Adhesives play an integral role in day-to-day military operations both on the battlefield and in the triage tent. Unfortunately, most adhesives lose their strength when exposed to water and aqueous solutions. Hydration compromises the intimate intermolecular contacts between the surface and adhering agent and promotes dissolution that results in partial or complete bonding failure [1].
L-DOPA: Sticky When Wet
To solve this problem, researchers have turned their attention to the remarkable adhesive properties of mussels, tunicates, barnacles, and tube worms [2, 3]. These organisms cling to wet rocks through the action of specialized proteins rich in the amino acid L-3,4-dihydroxyphenylalanine, or L-DOPA.
L-DOPA is a non-canonical amino acid produced in a variety of organisms, including humans. This amino acid is special in that it can form two hydrogen bonds, oxidatively cross-link, and coordinate transition metal ions like Fe3+ on the surface of rocks [4]. These synergistic interactions are too weak to adhere one molecule of L-DOPA to a target surface. However, the collective action of multiple L-DOPA molecules produces a strong adhesive force that can be exploited to design underwater adhesives [5].
State of the Art
The incorporation of L-DOPA-like molecules into synthetic polymers is the current strategy toward realizing water-resistant glues. In 2016 and 2017, the U.S. Office of Naval Research awarded funding to Dr. Bruce Lee (Michigan Technological University) and Dr. Johnathan Wilkner (Purdue University) to design underwater adhesives that may be used to install sensors and devices to the hulls of ships and submarines [6, 7].
The Wilkner laboratory synthesized a series of poly(catechol-styrene) polymers to mimic the chemistry of natural L-DOPA biopolymers and assessed their adhesion properties [7]. To do this, the researchers applied the polymers between two polished aluminum plates and submerged the plates in artificial seawater. After curing underwater, the plates were removed from the tank and immediately pulled apart by a materials testing system to quantify adhesion strength. It was found that higher molecular weight polymers had superior adhesive properties over lower molecular weight polymers and that the best biomimetic polymer outperformed several commercially available glues including Gorilla Glue® and Super Glue® (see Figure 1). Interestingly, adhesion was better in salt water than de-ionized water, suggesting an important role for ionic interactions [7].
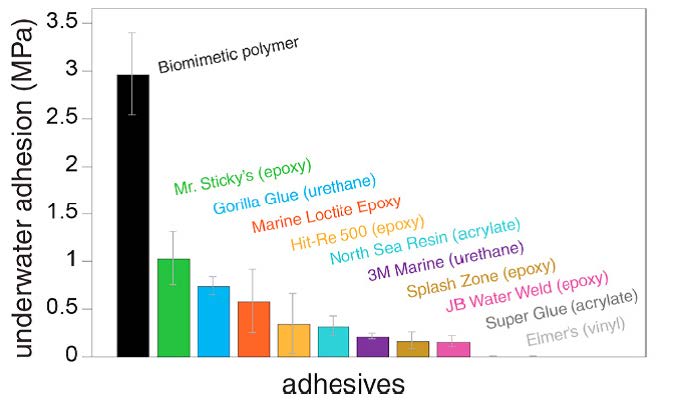
Figure 1. Bonding properties of biomimetic poly(catechol-styrene) polymers prepared by Wilker et al. compared to that of commercially available glues. Lap shear joints were between polished aluminum substrates. Adapted with permission from ACS Applied Materials & Interfaces, 9(8), 7866-7872. Copyright (2017) American Chemical Society.
In late 2017, the Yoshie laboratory developed next-generation underwater adhesives inspired by tunicates rather than mussels [8]. Their synthetic polymers adhered to all tested surfaces including metal, glass, plastic, wood, and pig skin, and were seven times stronger than mussel-inspired polymers. The main advantage of these particular polymers is the presence of a gallol moiety in lieu of catechol which enhances several non-covalent interactions to promote further adhesion [8]. This material remains one of the strongest underwater adhesives to date.
Conclusions
Nature continues to be a rich source of inspiration for the creation of novel underwater adhesives. Mussels must withstand the forces of pounding water that threaten to dislodge them from their rocky food source. The molecular mechanisms responsible for their adhesive properties can be incorporated into synthetic polymers that rival the strength of commercial glues.
The U.S. Office of Naval Research has a keen interest in the development of such products for use in underwater electronics and other potential applications that can advance military operations in damp or underwater environments. Water-resistant adhesives may also find complementary applications in wound healing and surgery to mend injuries on the battlefield.
REFERENCES
1. Wilker, J. J. (2015). Positive charges and underwater adhesion. Science, 349(6248), 582-583. doi:10.1126/science.aac8174
2. Wilker, J. J. (2010). Marine bioinorganic materials: mussels pumping iron. Current Opinion in Chemical Biology, 14(2), 276-283. doi:10.1016/j.cbpa.2009.11.009
3. Stewart, R. J., Ransom, T. C., & Hlady, V. (2011). Natural Underwater Adhesives. Journal of polymer science. Part B, Polymer Physics, 49(11), 757-771.doi:10.1002/polb.22256
4. Wilker, J. J. (2014). Self-healing polymers: Sticky when wet. Nat Mater, 13(9), 849-850. doi:10.1038/nmat4070
5. Silverman, H. G., & Roberto, F. F. (2007). Understanding marine mussel adhesion. Marine Biotechnology (New York, N.Y.), 9(6), 661-681. doi:10.1007/s10126-007-9053-x
6. Duffie, W., Jr. (2016, November). Mussel Power: ONR Researches Underwater Glue. Office of Naval Research. Retrieved from https://www.onr.navy.mil/en/Media-Center/Press-Releases/2016/Underwater-Glue-And-Mussels
7. North, M. A., Del Grosso, C. A., & Wilker, J. J. (2017). High Strength Underwater Bonding with Polymer Mimics of Mussel Adhesive Proteins. ACS Applied Materials & Interfaces, 9(8), 7866-7872. doi:10.1021/acsami.7b00270
8. Zhan, K., Kim, C., Sung, K., Ejima, H., & Yoshie, N. (2017). Tunicate-Inspired Gallol Polymers for Underwater Adhesive: A Comparative Study of Catechol and Gallol. Biomacromolecules, 18(9), 2959-2966. doi:10.1021/acs.biomac.7b00921


