Driven by their growing usefulness for fitness tracking, medical surveillance, and physiological status monitoring, wearable devices have received significant interest from academia, industry, and the Department of Defense (DoD) [1]. As the underlying technology has improved, potential military applications for what is known as real-time physiological status monitoring (RT-PSM) have widened in scope [2].
Research conducted at the U.S. Army Research Institute of Environmental Medicine contends that RT-PSM can allow for military planners and commanders to prevent warfighter exhaustion through early detection of high stress loads (thermal work-strain), provide triage options by detecting warfighter injury during a mission, and improve the efficacy of warfighter training through energy expenditure monitoring during drills [2]. As Dr. Matt Coppock, chemist and team lead for the U.S. Army Combat Capabilities Development Command’s Army Research Laboratory, noted in May 2019, “It can be envisioned that real-time health and performance monitoring” can help to optimize “individual to squad execution in multifaceted operational environments [3].”
Current wearables on the market are typically rigid—which is not ideal. Wearable sensors can achieve the best signal-to-noise ratio and achieve their maximum potential when constructed to maintain constant, conformal contact with the skin during body movements. A truly wearable device for health assessment and wireless physiological activity monitoring should be stretchable, mechanically robust and electrically stable under repeated loading, and mechanically unperceivable to the user. This is a difficult challenge, as human skin can be elastically stretched up to 15%, and the strain involved during daily motions can reach 100% for skin regions with wrinkles and creases (e.g., finger knuckles) [4]. It is particularly challenging when monitoring active warfighters, due to intensive body motions involved, and requirements for unobtrusiveness and comfortableness.
Wearable devices should be built upon stretchable substrates, such as elastomers or textiles, to achieve high levels of stretchability. There are two different, but complementary, approaches to developing stretchable electronics: top-down and bottom-up. In the topdown approach, thin films and ribbons are fabricated using conventional microfabrication techniques. Since elastomers are mostly incompatible with the microfabrication process, a transfer-printing step is required after patterning [5]. Geometrical designs are often required to introduce deformable structures to improve its stretchability [6].
Alternatively, the bottom-up approach uses synthesized nanomaterials to fabricate the stretchable devices due to their excellent mechanical compliance and large surface area. This approach is compatible with low-cost processes like solution-based methods and rollto-roll printing techniques. Wearable devices based on a variety of nanomaterials have been demonstrated, including metallic nanoparticles/ nanowires (e.g., silver nanoparticles [AgNPs], silver nanowires [AgNWs], gold nanoparticles [AuNPs]), carbon-based nanomaterials (e.g., carbon nanotubes [CNTs], graphene, graphene oxide [GO]), and transition-metal dichalcogenides [7–10].
With these devices, it is now feasible to capture the physical parameters (e.g., strain, tactile, temperature, and biopotential) [11–13], and chemical parameters (e.g., glucose, lactates, ions, electrolytes, and pH) from the human body [14, 15], as well as environmental parameters (e.g., ultraviolet and gas) from its surroundings [7, 16]. Our group has been developing various wearable devices using solution-based fabrication processes, including sensors to measure strain [16, 17], pressure [18], touch [18, 19], temperature [20], hydration [21], and biopotential signals [21–23]; wearable heaters [22]; and stretchable antenna [24]. The building block common to each application is AgNWs, owing to their simple synthesis and processing methods, and high conductivity and ductility (i.e., large failure strain). These AgNW-based wearable devices can achieve robust performance and reliability under highstrain and high-repetition conditions—such as those experienced by warfighters in training or operations.
Silver Nanowire-based Wearable Strain Sensors
Strain sensing is necessary to enable several applications of wearable electronics, including activity tracking, sports performance monitoring, and human-machine interfaces [25]. For example, strain sensors can help quantify body motions (e.g., angle, speed, acceleration) during exercise in order to improve posture, evaluate athletic performance, prevent injuries, and facilitate rehabilitation.
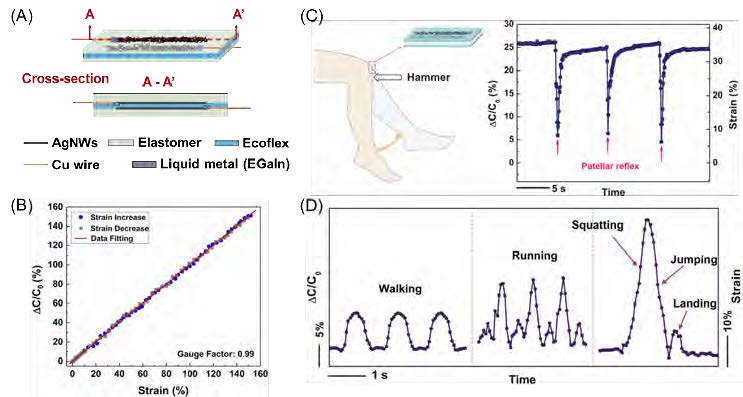
Figure 1. (A) Schematic illustration of the fabricated capacitive strain sensor. (B) Relative capacitance change as a function of tensile strain for stretching and releasing. Reproduced with permission [15]. Copyright 2018, IEEE. (C) Capacitance change and strain associatedwith knee motions in patellar reflex. (D) Relative capacitance change and strain associated with various human motions. (C, D) Reproduced with permission [16]. Copyright 2013, The Royal Society of Chemistry.
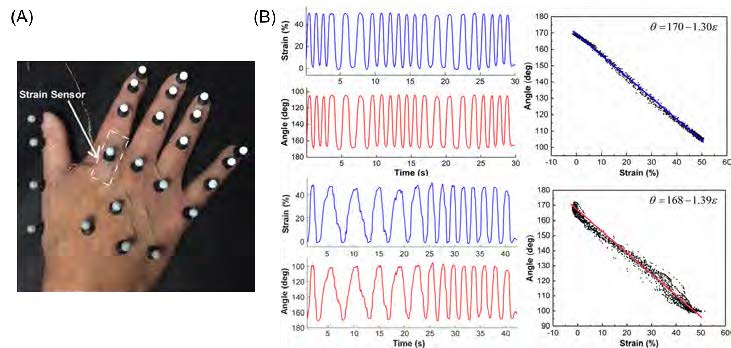
Figure 2. (A) A skin-like strain sensor and reflective markers attached onto the hand of a stroke patient. (B) Comparisons between the strain measured from the strain sensors (blue) and the angle measured from the IR cameras (red) for a healthy control (top panel) and astroke patient (bottom panel). (A, B) Reproduced with permission [15]. Copyright 2018, IEEE.
Conventional strain gauges typically have a strain operating range of less than 5%, and can be used for stress analysis such as flight testing or structural integrity monitoring of critical infrastructure [26]. However, they cannot meet the large-strain requirement (> 50%) for wearable electronics.
Strain sensing techniques such as fiber Bragg grating, Raman shift, piezoelectricity, and triboelectricity are not practical for wearable applications largely due to their poor stretchability and need for sophisticated measurement equipment [27]. Both resistive and capacitive sensing mechanisms have been adopted to develop wearable strain sensors [7]. The resistive sensing method is advantageous in terms of its sensitivity and simplicity of data acquisition, but it suffers from poor linearity and large hysteresis.
On the other hand, capacitive strain sensing offers excellent stretchability, linearity, and low hysteresis. A wide range of stretchable and conductive materials can serve as the electrodes, including CNTs, AgNPs, graphene, and metallic nanowires—among which AgNWs exhibit the best conductivity for a given density [8].
As illustrated in Figure 1A, the strain sensor is essentially a stretchable capacitor, where the top and bottom electrodes were fabricated by embedding AgNWs just below the surface of an elastomer (polydimethylsiloxane (PDMS) [18] or Ecoflex [17]). To serve as the dielectric, a thin layer of highly stretchable Ecoflex was sandwiched between the two electrodes. Under a tensile strain, the length of the capacitor increases as the width and the distance between the two electrodes decrease, resulting in a capacitance increase (see Figure 1B). The gauge factor (relative capacitance divided by the mechanical strain) for the strain sensor with AgNW/Ecoflex electrodes is close to 1 for strain of up to 150%, which is sufficient for monitoring human motions [17]. In addition to a constant gauge factor over a wide strain range, our strain sensor demonstrated a fast response time, low hysteresis, and skin-like mechanical properties (Young’s modulus of 96 kPa).
Preliminary experiments have revealed good wearability and large-deformation sensing capabilities. These results came from mounting the capacitive strain sensors onto a human body to monitor skin deformations associated with finger flexing (Figure 2), knee motions in the patellar reflex (Figure 1C), and other motions, such as walking, running, and jumping from squatting (Figure 1D) [18].
Recently, our group cross-validated the accuracy of our strain sensors by comparing them against conventional optical motion tracking systems using reflective markers and infrared (IR) cameras.
Figure 2A shows the experimental setup, where both the strain sensors and reflective markers were attached to the hand of a stroke patient. The strain associated with finger flexing was monitored using the attached strain sensor, and the finger bending angle was captured by the IR cameras simultaneously. Figure 2B shows excellent correlation between the two methods for tracking the joint motions of a stroke survivor and a healthy control. In addition, it shows that the strain sensor possesses excellent resolution to capture the details of the jerky motions of the stroke patient.
Silver Nanowire-based Wearable Hydration Sensors
Hydration levels regulate key body functions such as core temperature, blood pressure, and heart rate. Dehydration can be a serious risk factor for warfighters, as well as a symptom of potential ailments like skin disease, kidney stones, and gastroenteritis [28, 29]. Real-time tracking of hydration levels can benefit those working in extreme conditions, and help evaluate the effectiveness of medical therapies [30]. Most portal hydration monitors on the market place electrodes onto the skin to measure changes in the skin’s conductance, capacitance, and impedance. However, such planar electrodes are rigid and need to be manually pressed against the skin for reliable reading. The Rogers research group has made recent progress in this area, demonstrating a series of photolithographically patterned epidermal devices in serpentine layouts for skin-attachable hydration sensing [30, 31].
To enable a conformal electrical and mechanical interface to the skin, we based our hydration sensors on AgNW/PDMS conductors, where AgNWs were inlaid under the surface of PDMS [32]. The AgNW/PDMS conductors combine the superior conductivity of silver with the enhanced stretchability of nanomaterials and polymers to achieve highly conductive electrodes which can maintain good conductivity up to 50% tensile strain [33]. The AgNW/ PDMS conductors were patterned into interdigitated electrodes to facilitate the impedance measurement (see Figure 3A).
Upon placing the hydration sensor onto the skin, impedance (which is dependent on hydration levels) can be measured from the two terminals. The AgNW hydration sensor was calibrated against a commercial hydration monitor (Moisture Meter D (MMD), Delfin Tech). As shown in Figure 3B, when the hydration level (MMD reading) increases, the impedance measured from the AgNW sensor decreases.
To achieve a better wearability form factor, the AgNW sensor was combined with a network analyzer chip, button cell battery, ultralow power microprocessor, and Bluetooth transmitter, and integrated into a flexible wristband as a watch-type hydration monitor (see Figure 3C). The resulting wearable hydration monitor is compliant, wireless, and can be continuously worn on the skin for long periods of time without degrading its performance.
Silver Nanowire-based Dry Biopotential Electrodes
Biopotential electrodes capture bioelectric variations and electrophysiological signals in tissues. Representative electrophysiological signals include electrocardiogram (ECG), electromyogram (EMG), electroencephalogram, and electrooculogram, which reflect the electrical activity of the heart, muscle, brain, and eyes, respectively. Conventionally pre-gelled Ag/AgCl wet electrodes are used to capture the electrical signals, where conductive gel is used to establish a reliable electrode-skin interface.
Wet electrodes are flexible and wearable; however, the gel dries over time, leading to degraded signal quality. The dehydration of the conductive gel requires the gel to be re-applied, which is inconvenient and sometimes infeasible. Moreover, the gel and adhesive can potentially trigger skin irritations [32, 34]. Therefore, gel-free (dry) electrodes are greatly needed. Metal thin films and nanomaterials, including metallic nanomaterials and carbon-based nanomaterials, have been used to develop biocompatible and compliant dry electrodes for long-term monitoring [32].
Highly conductive and stretchable conductors made of AgNWs embedded in PDMS matrix were used for electrophysiological sensing without the use of conductive gel [23]. The positive and negative electrodes were placed on the left and right arms using Velcro straps (see Figure 4A), and the ground electrode was placed on the right leg. Reliable electrode-skin impedance is crucial for acquiring a high signal-to-noise ratio and low motion artifact. Here, the good compliance of the AgNW/PDMS electrode allows for low electrode-skin impedance without the gel and good contact with skin during body motions. As shown in Figure 4B, the AgNW/PDMS dry electrode performed as well as the pre-gelled Ag/AgCl electrodes when the subject was resting, swinging their arms, and jogging. Each wave of the ECG signal (i.e., the P wave, QRS complex, and T wave) can be clearly identified from the ECG waveforms.
Heart rate can also be readily extracted from the R-R interval of ECG signals. As cardiovascular disease is the leading cause of death in the U.S., continuous ECG monitoring in wearable form factors could significantly improve public health outcomes and decrease overall health care costs. In addition to ECG, the electrodes can also be used for surface EMG measurements. For example, AgNW/PDMS electrodes were placed on the right extensor digitorum communis and used to capture muscle activities during wrist extension-contractions [23].
The strain sensors, hydration sensors, and dry electrodes discussed above were fabricated via solution-based coating methods (i.e., drop casting), with the use of a mask to generate patterns. Additionally, we have demonstrated the electrohydrodynamic (EHD) printing of AgNWs onto flexible and stretchable substrates [22]. As illustrated in Figure 4C, EHD printing systems typically include a pneumatic dispensing system, a voltage supplier, and a precision three-axis translation stage. To make the ink for EHD printing, 4 wt% poly(ethylene oxide) (PEO) was added to a 15 mg/ml AgNW/water solution to adjust the viscosity of the ink. To enable the best printing results, the inner diameter of the nozzle was set at 150 μm, and the outer diameter of the nozzle at 250 μm. The printing voltage was set at 1500 V, with a standoff distance (distance between printing head and substrate) of 75 μm, and back pressure of 0.4 psi.
With EHD printing, AgNW inks can be printed onto various substrates without masks, including PDMS (dopamine treated), PET, glass, letter paper, nanofiber paper, polycarbonate filter, and nature rubber latex (i.e, lab-use gloves). The smallest linewidth was ~45 μm. After removing PEO with water and heat treatment, high conductivity of ~5.6×106 S/m was achieved. AgNWs were printed onto PDMS substrate following a Greece Cross pattern for ECG sensing (see Figure 4E). This fractal inspired pattern ensures good mechanical stretchability and large area coverage [35]. ECG recordings were successfully acquired by placing two AgNW/PDMS fractal electrodes on the chest. Comparable signal quality with commercial pre-gelled electrodes has been demonstrated.
Silver Nanowire-based Wearable Temperature Sensors
Body temperature is one of the most important vital signals, and it correlates with illnesses such as fever, heat stroke, and infection [7]. Wearable temperature monitoring requires good stretchability, fast response, a wide sensing range (25–50 °C), and high precision (±0.1 °C in the range of 37–39 °C and ±0.2 °C for below 37 °C and above 39 °C) [6, 35]. The main challenge associated with wearable temperature sensors is the crosstalk between temperature and strain during body movements. It is difficult to differentiate the relative contributions of temperature and strain to the overall change in electrical signals. To overcome this problem, a stretchable temperature sensor that is insensitive to strain is required.
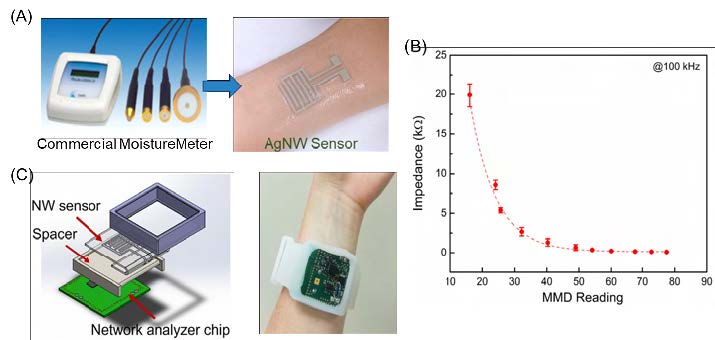
Figure 3. (A) Images showing the commercial hydration monitor (left) and the AgNW sensor worn on the wrist (right). (B) Skin impedance measured from the AgNW hydration sensor as a function of the hydration level measured from the commercial hydration monitor. (C)Schematics showing the PCB, 3D printed spacer, AgNW skin hydration sensor, and encasing (left). The hydration monitor worn on the wrist like a watch (right). (A–C) Reproduced with permission [19]. Copyright 2017, John Wiley and Sons.
Our group developed a stretchable thermoresistive temperature sensor based on AgNW/ polyimide (PI) composite [20]. The temperature sensor was patterned with a Kirigami structure, where cuts were introduced to enable out-ofplane deformations during stretching to minimize the local strain in the AgNW/PI thin film (see Figure 5A). The Kirigami structure also makes the temperature sensor vapor-permeable to prevent heat and sweat accumulation, improving the comfort for long-term wear.
Figure 5B shows the relative resistance change of the AgNW/PI temperature sensor during loading and unloading, up to 100% strain. Owing to the introduction of the Kirigami structure, the variation of the resistance was within 0.05%, indicating negligible sensitivity to strain. Temperature coefficient of resistance (TCR), defined as follows, is commonly used to describe the sensitivity of a thermoresistive temperature sensor:
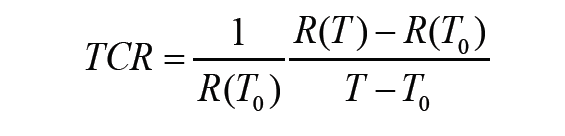
where R(T0) and R(T) are the resistance at temperatures T0 and T, respectively.
The TCR of the AgNW/PI temperature sensors increases with NW density (from 0.26 to 2.05 nanowires per μm2 ) and annealing temperature (up to 200 °C). As shown in Figure 5C, for the AgNW network density of 2.05 per μm2 after 200 °C annealing, the calculated TCR was 3.32×10-3/°C and the sensitivity was 0.47 Ω/°C over the temperature range from 25 °C to 60 °C. Negligible difference in the TCR and sensitivity was observed with 100% tensile strain and without strain. To demonstrate practical wearable applications, the AgNW/PI temperature sensor was attached onto the skin near the biceps to monitor the temperature change during exercise (see Figure 5D). The temperature change was also monitored using a commercial IR thermometer for comparison. Good correlation was achieved for temperature variations recorded from the wearable AgNW/PI temperature sensor and the IR thermometer.
Silver Nanowire-based Wearable Heaters
Our group has also worked to develop improved, highly flexible wearable heaters. Wearable heaters can protect warfighters from extreme cold-weather climates and facilitate the recovery of joint fatigue and injuries. Heat improves blood flow, alleviates pain, relieves muscle spasms, decreases joint stiffness, and reduces inflammation [37, 38]. Conventional wearable heating elements include heat packs and resistive heating wraps. Heat packs are typically bulky and thick, and the temperature is noncontrollable and nonuniform, causing discomfort [39, 40]. Joule-heating wraps offer well-controlled heating temperature; however, due to their low flexibility, they fail to conform to the curvilinear surface of the skin. Commercially-used electrothermal materials, such as ferro chromium-based alloys, are challenged by high rigidity and low heating efficiency [41]. Indium tin oxide (ITO) has been the dominant electrode material due to its good electrical conductivity. With rising indium costs and other limitations in mind—including slow thermal response, harsh processing conditions, and dramatically deteriorated conductivity under strain—alternative conductive materials are in demand to replace ITO for heating applications [8].
AgNWs were adopted to develop wearable heaters. Their conductivity guarantees a low actuation voltage, and mechanical compliance enables good robustness against mechanical deformations. AgNWs were directly printed by EHD onto flexible substrate with Peano curve fractal patterns [35]. As depicted in the IR thermal images of Figure 6A, the 6×6 mm heater can provide a temperature up to ~160 °C at the voltage of 25 V, with maximum heating and cooling rate of 21 and 29 °C s-1, respectively.
To demonstrate wearability, the heater was mounted onto the thumb area (see Figure 6B). The heater maintained stable temperature under deformations caused by finger movements, illustrating its reliable performance during body motions. Besides applications for thermotherapy, wearable heaters can be further integrated with thermo-responsive drug release systems for wearable drug delivery, as demonstrated by the Kim research group [42, 43]. When RTPSM monitors detect an injury or other medical emergency, wearable heaters can potentially be used to administrator therapeutic therapies through thermotherapy or thermo-responsive drug delivery.
Wearable heaters can actively protect warfighters from extreme cold environments while maintaining their body temperature for good performance. The integrated wearable therapy components, together with the wearable sensors, can provide timely and closed-loop healthcare, which is especially beneficial in life-threatening situations.
Conclusion
Wearable, wireless, and multimodal sensors provide real-time physiological parameters (e.g., temperature, hydration, ECG, and motions) and environmental data (e.g., temperature, humidity, and pressure) for activity monitoring, performance tracking, warfighter fatigue detection, and strategic planning [1].
As discussed in this article, our group developed a variety of AgNW-enabled wearable devices—all fabricated through low-cost, solution-based processes. As these technologies continue to improve, the data collected by RT-PSM sensors could be used to develop what the U.S. Army Research Institute of Environmental Medicine calls a “soldier readiness score”—an index comprised of musculoskeletal fatigue limits, thermal work-strain loads, and mission-specific physiological status parameters (e.g., pulmonary threats, hypoxia) [2]. Active analysis of such readiness scores improves unit readiness and aids commanders in matching individual operators to the needs and capabilities required by specific missions [2].
The unprecedented combination of mechanical stretchability and electrical conductivity of AgNW/elastomer nanocomposites allows for excellent wearability and robust performance, even under repeated body movements. To promote the practical applications of AgNW based wearable devices, it is important to scale up the fabrication of wearable devices and improve reliability over long periods of use, where ongoing efforts are currently underway [21, 43, 44].
References
1.Majidi, C. (2018). Electronic stickers for wireless physiological monitoring in a tactical environment. Journal of the Homeland Defense & Security Information Analysis Center, 5(4). Retrieved from https://www.hdiac.org/wp-content/uploads/Electronic_ Stickers_for_Wireless_Physiological_Monitoring_V5I4.pdf
2. Friedl, K. E. (2018). Military applications of soldier physiological monitoring. Journal of Science and Medicine in Sport, 21, 1147– 153. doi:10.1016/j.jsams.2018.06.004
3. U.S. Army Combat Capabilities Development Command Army Research Laboratory Public Affairs. (2019, May 1). Wearable sensors could leverage biotechnology to monitor personal, environmental data. Retrieved from https://www.army.mil/article/221184
4. Webb, R. C., Bonifas, A. P., Behnaz, A., Zhang, Y., Yu, K. J., Cheng, H., . . . & Rogers, J. A. (2013). Ultrathin conformal devices for precise and continuous thermal characterization of human skin. Nature Materials, 12(10), 938–944. doi:10.1038/nmat3755
5. Rogers, J. A., Someya, T., & Huang, Y. (2010). Materials and mechanics for stretchable electronics. Science, 327(5973), 1603– 1607. doi:10.1126/science.1182383 6. Wang, C., Wang, C., Huang, Z., & Xu, S. (2018). Materials and structures toward soft electronics. Advanced Materials, 30(50), 1801368. doi:10.1002/adma.201801368
7. Yao, S., Swetha, P., & Zhu, Y. (2018). Nanomaterial-enabled wearable sensors for healthcare. Advanced Healthcare Materials, 7(1), 1700889. doi:10.1002/adhm.201700889
8. Yao, S., & Zhu, Y. (2015). Nanomaterial-enabled stretchable conductors: Strategies, materials and devices. Advanced Materials, 27(9), 1480–1511. doi:10.1002/adma.201404446
9. Gong, S., & Cheng, W. (2017). One-dimensional nanomaterials for soft electronics. Advanced Electronic Materials, 3(3), 1600314. doi:10.1002/aelm.201600314
10. Trung, T. Q., & Lee, N. E. (2017). Recent progress on stretchable electronic devices with intrinsically stretchable components. Advanced Materials, 29(3), 1603167. doi:10.1002/adma.201603167
11. Misra, V., Bozkurt, A., Calhoun, B., Jackson, T., Jur, J. S., Lach, J., . . . , & Zhu, Y. (2015). Flexible technologies for self-powered wearable health and environmental sensing. Proceedings of the IEEE, 103(4), 665–681. doi:10.1109/JPROC.2015.2412493
12. Trung, T. Q., & Lee, N. E. (2016). Flexible and stretchable physical sensor integrated platforms for wearable human-activity monitoring and personal healthcare. Advanced Materials, 28(22), 4338–4372. doi:10.1002/adma.201504244
13. Yeo, J. C., & Lim, C. T. (2016). Emerging flexible and wearable physical sensing platforms for healthcare and biomedical applications. Microsystems & Nanoengineering, 2, 16043. doi:10.1038/micronano.2016.43
14. Bandodkar, A. J., Jeerapan, I., & Wang, J. (2016). Wearable chemical sensors: Present challenges and future prospects. ACS Sensors, 1(5), 464–482. doi:10.1021/acssensors.6b00250 15. Bandodkar, A. J., & Wang, J. (2014). Non-invasive wearable electrochemical sensors: A review. Trends in Biotechnology, 32(7), 363–371. doi:10.1016/j.tibtech.2014.04.005
16. Tricoli, A., Nasiri, N., & De, S. (2017). Wearable and miniaturized sensor technologies for personalized and preventive medicine. Advanced Functional Materials, 27(15), 1605271. doi: 10.1002/adfm.201605271
17. Yao, S., Vargas, L., Hu, X., & Zhu, Y. (2018). A novel finger kinematic tracking method based on skin-like wearable strain sensors. IEEE Sensors Journal, 18(7), 3010–3015. doi:10.1109/JSEN.2018.2802421
18. Yao, S., & Zhu, Y. (2014). Wearable multifunctional sensors using printed stretchable conductors made of silver nanowires. Nanoscale, 6(4), 2345–2352. doi:10.1039/ C3NR05496A 19. Cui, Z., Poblete, F. R., Cheng, G., Yao, S., Jiang, X., & Zhu, Y. (2014). Design and operation of silver nanowire based flexible and stretchable touch sensors. Journal of Materials Research, 30(1), 79–85. doi:10.1557/jmr.2014.347
20. Cui, Z., Poblete, F. R., & Zhu, Y. (2019). Tailoring temperature coefficient of resistance of silver nanowire nanocomposite and application as stretchable temperature sensor. ACS Applied Materials & Interfaces. doi:10.1021/acsami.9b04045
21. Yao, S., Myers, A., Malhotra, A., Lin, F., Bozkurt, A., Muth, J. F., & Zhu, Y. (2017). A wearable hydration sensor with conformal nanowire electrodes. Advanced Healthcare Materials, 6(6), 1601159. doi:10.1002/adhm.201601159
22. Cui, Z., Han, Y., Huang, Q., Dong, J., & Zhu, Y. (2018). Electrohydrodynamic printing of silver nanowires for flexible and stretchable electronics. Nanoscale, 10(15), 6806–6811. doi:10.1039/C7NR09570H
23. Myers, A. C., Huang, H., & Zhu, Y. (2015). Wearable silver nanowire dry electrodes for electrophysiological sensing. RSC Advances, 5(15), 11627–11632. doi:10.1039/C4RA15101A
24. Song, L., Myers, A. C., Adams, J. J., & Zhu, Y. (2014). Stretchable and reversibly deformable radio frequency antennas based on silver nanowires. ACS Applied Materials & Interfaces, 6(6), 4248–4253. doi:10.1021/am405972e
25. Amjadi, M., Kyung, K. U., Park, I., & Sitti, M. (2016). Stretchable, skin-mountable, and wearable strain sensors and their potential applications: A review. Advanced Functional Materials, 26(11), 1678–1698. doi:10.1002/adfm.201504755
26. Dally, J. W., Riley, W. F., & McConnell, K. G. (1993). Instrumentation for Engineering Measurements (2rd ed.). New York, NY: Wiley & Sons.
27. Park, J., You, I., Shin, S., & Jeong, U. (2015). Material approaches to stretchable strain sensors. ChemPhysChem, 16(6), 1155–1163. doi:10.1002/cphc.201402810
28. Sheehy, C. M., Perry, P. A., & Cromwell, S. L. (1999). Dehydration: Biological considerations, age-related changes, and risk factors in older adults. Biological Research for Nursing, 1(1), 30–37. doi:10.1177/109980049900100105
29. Chan, J., Knutsen, S. F., Blix, G. G., Lee, J. W., & Fraser, G. E. (2002). Water, other fluids, and fatal coronary heart disease: The adventist health study. American Journal of Epidemiology, 155(9), 827–833. doi:10.1093/aje/155.9.827
30. Huang, X., Yeo, W.-H., Liu, Y., & Rogers, J. A. (2012). Epidermal differential impedance sensor for conformal skin hydration monitoring. Biointerphases, 7(1), 52. doi:10.1007/s13758-012-0052-8
31. Krishnan, S., Shi, Y., Webb, R. C., Ma, Y., Bastien, P., Crawford, K. E., . . ., & Rogers, J. A. (2017). Multimodal epidermal devices for hydration monitoring. Microsystems & Nanoengineering, 3, 17014. doi:10.1038/micronano.2017.14
32. Yao, S., & Zhu, Y. (2016). Nanomaterial-enabled dry electrodes for electrophysiological sensing: A review. JOM, 68(4), 1145–1155. doi:10.1007/s11837-016-1818-0
33. Xu, F., & Zhu, Y. (2012). Highly conductive and stretchable silver nanowire conductors. Advanced Materials, 24(37), 5117–5122. doi: 10.1002/adma.201201886
34. Searle, A., & Kirkup, L. (2000). A direct comparison of wet, dry and insulating bioelectric recording electrodes. Physiological Measurement, 21(2), 271. doi:10.1088/0967- 3334/21/2/307 35. Fan, J. A., Yeo, W. H., Su, Y., Hattori, Y., Lee, W., Jung, S. Y., . . ., & Rogers, J. A. (2014). Fractal design concepts for stretchable electronics. Nature Communications, 5, 3266. doi:10.1038/ncomms4266
36. Li, Q., Zhang, L. N., Tao, X. M., & Ding, X. (2017). Review of flexible temperature sensing networks for wearable physiological monitoring. Advanced Healthcare Materials, 6(12), 1601371. doi:10.1002/adhm.201601371
37. Dehghan, M., & FarahbOD, F. (2014). The efficacy of thermotherapy and cryotherapy on pain relief in patients with acute low back pain, a clinical trial study. Journal of Clinical and Diagnostic Research, 8(9), LC01. doi:10.7860/JCDR/2014/7404.4818
38. Malanga, G. A., Yan, N., & Stark, J. (2015). Mechanisms and efficacy of heat and cold therapies for musculoskeletal injury. Postgraduate Medicine, 127(1), 57–65. doi:10.1 080/00325481.2015.992719
39. Choi, S., Park, J., Hyun, W., Kim, J., Kim, J., Lee, Y. B., . . ., & Hyeon, T. (2015). Stretchable heater using ligand-exchanged silver nanowire nanocomposite for wearable articular thermotherapy. ACS Nano, 9(6), 6626– 6633. doi:10.1021/acsnano.5b02790
40. Li, Y.-Q., Zhu, W.-B., Yu, X.-G., Huang, P., Fu, S.-Y., Hu, N., & Liao, K. (2016). Multifunctional wearable device based on flexible and conductive carbon sponge/polydimethylsiloxane composite. ACS Applied Materials & Interfaces, 8(48), 33189–33196. doi:10.1021/acsami.6b11196
41. Lin, S.-Y., Zhang, T.-Y., Lu, Q., Wang, D.- Y., Yang, Y., Wu, X.-M., & Ren, T.-L. (2017). High-performance graphene-based flexible heater for wearable applications. RSC Advances, 7(43), 27001–27006. doi:10.1039/c7ra03181e
42. Lee, H., Song, C., Hong, Y. S., Kim, M. S., Cho, H. R., Kang, T., . . ., & Kim, D.-H. (2017). Wearable/disposable sweat-based glucose monitoring device with multistage transdermal drug delivery module. Science Advances, 3(3), e1601314. doi:10.1126/sciadv.1601314
43. Lee, H., Choi, T. K., Lee, Y. B., Cho, H. R., Ghaffari, R., Wang, L., . . ., & Kim, D.-H. (2016). A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nature Nanotechnology, 11, 566–572. doi:10.1038/ nnano.2016.38
44. Huang, Q., & Zhu, Y. (2018). Gravure printing of water-based silver nanowire ink on plastic substrate for flexible electronics. Scientific Reports, 8(1), 15167. doi:10.1038/s41598-018-33494-9
45. Huang, Q., & Zhu, Y. (2019). Printing conductive nanomaterials for flexible and stretchable electronics: A review of materials, processes, and applications. Advanced Materials Technologies, 1800546. doi:10.1002/admt.201800546


