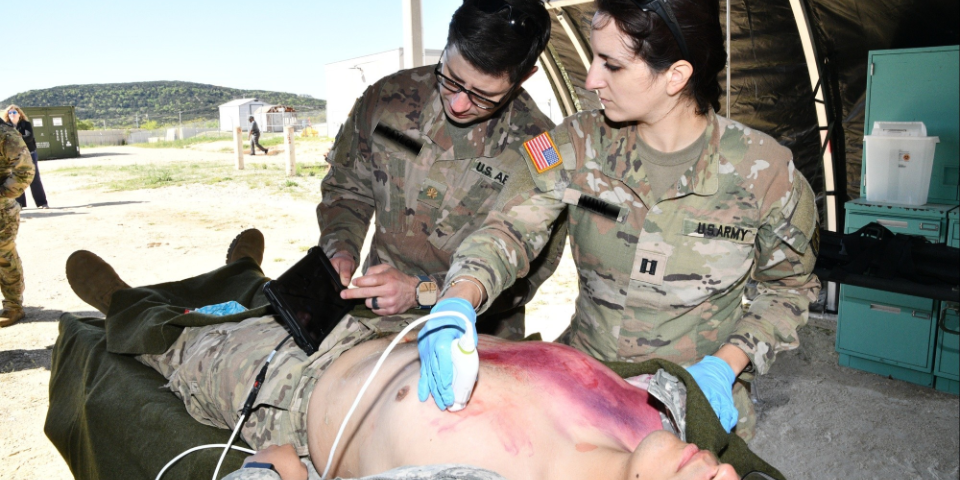
JOINT BASE SAN ANTONIO, TX – The U.S. Army Medical Evaluation Test Activity (USAMTEAC) recently conducted an operational test of three ultrasound field portable devices. The test was conducted for the U.S. Army Medical Materiel Development Activity (USAMMDA)/Warfighter Expeditionary Medicine and Treatment (WEMT) Project Management Office to evaluate how the devices perform in a simulated operational environment. Currently, the U.S. Army does not have portable ultrasound devices for deployed medicine. One of the three devices being tested will be selected by USAMMDA/WEMT to provide soldiers with a new capability for battlefield medicine.
The ultrasound field portable is a small, lightweight, portable, U.S. Food and Drug Administration-cleared ultrasound device. It is a new requirement to fill a capability gap for the Tactical Combat Medical Care, Austere Tactical Care, and Special Forces Tactical Medical Equipment Sets. The ultrasound device directs high-frequency sound waves at the internal body structures being examined. The reflected sounds, or echoes, are recorded to create an image that can be seen on a portable viewing screen. The sound waves are emitted and received from a small, hand-held probe and/or probes.
Army 65D Physician Assistant Maj. Maya Lowell with USAMTEAC described the benefit of the operational test. “The ultrasound is intended to go down to a Role 1/battalion aid station (BAS) and Role 2, so it’s important that we assess how the device performs in different environmental conditions. At the Role 1 where there currently is no imaging capability, the ultrasound may be able to identify several pathological processes such as internal bleeding. It’s a tool that someone at the Role 1 can use to determine how critical that patient is, what medical interventions they are going to need, and what evacuation category they should be.”