In 2007, the Department of Veterans Affairs implemented its National MRSA Prevention Initiative program to reverse skyrocketing health care costs and poor patient outcomes caused by the deadly bacteria methicillin-resistant Staphylococcus aureus, more commonly known as MRSA. Because of its resistance to common antibiotics, such as methicillin and related β-lactam compounds, the Centers for Disease Control and Prevention considers MRSA a serious threat to public health.
In 2011, MRSA infected 80,500 people, with nearly 1 in 7 (11,300) cases resulting in death. [1] MRSA is a major infective agent in the lung, at surgical sites and in the bloodstream. Hospital acquired MRSA infections increase health care costs [2] due to specialized laboratory testing needed to confirm the presence of drug resistant MRSA bacteria.
Because MRSA infections can occur in medical care facilities, the VA enacted stringent measures within NMPI to curtail disease transmission with specialized hygiene, sanitation and decontamination practices.
The VA has 153 medical centers with around 600,000 patient admissions each year. [3] The median length of stay is three days. [3] During a three-year period from 2007 to 2010, 13.6 percent of the 1.7 million patients screened for MRSA upon admission tested positive, whereas only 6.3 percent of patients tested positive for MRSA upon admission to a non-VA facility. [3] This demonstrates the increased the risk for acquiring a MRSA infection in a VA facility compared with a non-VA facility.
In addition, the highest risk lies within intensive care units. [3] Studies show that VA patients have a higher readmission rate if they have had MRSA infections. [4] One of the NMPI’s goals is to lower the rate of MRSA infection (this is an unspecified number because any reduction is better than no reduction), thereby lowering transmission rates within the health care setting. The outcome of these efforts manifests as a reduction in the rate of MRSA infection within VA patients admitted to the hospital. [5]
The rate of MRSA hospital-acquired infection within VA facilities was about 1.5 cases per 1000 patient-days in 2006, [3] which means there were approximately 2,700 MRSA infections during 1.8 million patient days in a VA health care setting in 2006. Compared to other infections, MRSA triples the per-patient hospital expenses (~$90,000/patient for MRSA hospital- acquired infections versus ~$30,000 for non-MRSA hospital-acquired infections). [3]
MRSA also impacts patients after they leave the hospital. Side-effects and morbidity from MRSA and its treatment double post-discharge costs (~$36,000 for MRSA hospital-acquired infections
versus $18,000 for non-MRSA hospital- acquired infections). [6] Using these estimates, the 2,700 hospital-acquired MRSA cases in 2006 increased health care costs by $210 million. In a thorough analysis of MRSA prevention and costs between 2007 and 2010, NMPI lowered hospital-acquired infections of MRSA within intensive care units by 70 percent (from 783 to 241) and cut non-ICU MRSA hospital-acquired infections in half. [6]
During the three-year analysis period, the program resulted in 2000 fewer MRSA hospital-acquired infections and lowered health care costs by $75 million. [6] These efforts are laudable and
demonstrate the impact of improving patient care practices and hospital procedures to lower MRSA infection rates. Another option to reduce MRSA infection rates is with improved antibiotics.
In 2011, the U.S. healthcare system prescribed 118 million courses of β-lactam antibiotics. [7] In their current form, however, these drugs do not stop MRSA infections. The inability to immediately
identify and treat infections from MRSA leads to morbidity, mortality and increased health care costs. [2,8] Delays in delivering effective anti-MRSA drugs can be overcome by screening– a daunting task that the VA has successfully employed, yet is burdensome on the U.S. healthcare system that deals with hundreds of millions of bacterial infection cases each year. [7]
The University of Oklahoma’s work is directed towards an alternative: improving first-line antibiotics to simultaneously kill both MRSA and the non-drug-resistant staph infection bacteria.
The vast majority of patients presenting with symptoms of bacterial infection are treated with inexpensive broad-spectrum β-lactam antibiotics. [7] Patient outcomes are positive, unless β-lactam
resistant bacteria, such as MRSA, are present. Surviving MRSA colonies invade host tissue to release toxins that cause tissue injury, leading to significant patient morbidity. In cases where the initial symptoms are attributed to MRSA, β-lactams are avoided in favor of more effective antibiotics given without delay. [9]
Most MRSA infections, however, are not diagnosed immediately. Instead, after initial infection symptoms arise, patients suffer while numerous first and second- line antibiotics are prescribed to no avail. [10] Upon the initial presentation of staph infection symptoms, a critical need exists for a first-line antibiotic to treat both MRSA and susceptible staph bacteria without the need diagnose MRSA and use more expensive antibiotics. [11] This requires a therapy that can block the function of penicillin binding protein 2a (PBP2a) and PBP4, the leading causes of β-lactam antibiotic resistance in MRSA infections. [12,13]
It may be possible to achieve this outcome with a discovery made at OU. [14] Low cost β-lactam antibiotics that kill non-resistant staph infection bacteria also prevent the growth of MRSA if administered with a readily available and inexpensive polymer: branched poly(ethylenimine), or BPEI. The goal is to develop β-lactam + BPEI combinations as a potential first-line antibiotic. This route to reduce morbidity, mortality and health care costs has the ability to restore anti-MRSA potency to obsolete FDA approved antibiotics, thereby improving the MRSA infection control aspects of NMPI in a cost-effective manner.
If MRSA is diagnosed or suspected, several antibiotics can be used (vancomycin, linezolid, daptomycin). To date, no MRSA strain is currently resistant to more than one of them. [15,16] People die from MRSA infections because MRSA was not initially suspected, and thus ineffective first-line antibiotics, usually β-lactams, are given. MRSA relies on PBP2a and PBP4 to survive in the presence
of β-lactam antibiotics. [12,13]
Only after morbidity from MRSA toxins are more effective antibiotics given and these life-saving medications become drugs of last resort. Unfortunately, in many cases, the effective drug is given
too late to prevent mortality. [2,15] Life threatening situations can be avoided with timely treatment. [17] New antibiotics, such as oxadiazoles, [18] tedizolid [19] and teixobactin, [20] will be new
drugs of last resort.
But, according to OU, these drugs are not likely to be first-line antibiotics due to their cost. Thus, they will reduce mortality rates but may not reduce morbidity. Likewise, they will not reduce healthcare costs incurred from long periods of hospitalization, sometimes in the intensive care unit, while giving drugs of last resort. [21-25] These new drugs of last resort are expensive, often require intravenous delivery and their use is often delayed until specialized laboratory testing confirms the presence of drug resistant bacteria. [2,10] As an alternative, the different approach brought forth by researchers at OU may be able to kill MRSA before its toxins cause widespread damage to tissue and endanger the patient’s life.
The approach utilizes a cationic polymer that disables resistance with MRSA, making it susceptible to low-cost penicillin-type β-lactam antibiotics. As scientific research moves forward, the goal for this technology is to lower health care costs, reduce tragic morbidity and save lives. As depicted in Figure 1, the bacterial cell wall is composed of peptidoglycan and wall teichoic acid. Susceptible S. aureus is killed by cellular lysis caused by two factors working together: 1) β-lactams that disable penicillin binding proteins that perform essential cell-wall crosslinking chemistry (See Figure 1B, 1G); and 2) activity of enzymes that degrade existing cell wall peptidoglycan. Methicillin, a β-lactam antibiotic, occupies the active site of penicillin binding proteins to prevent the enzyme’s cell wall synthesis function. MRSA uses a variant of PBP (PBP2a) that is not affected by β-lactams (See Figure 1C) and therefore peptidoglycan biosynthesis continues, allowing
MRSA to persist under antibiotic attack. This mechanism of antibiotic resistance is not perfect; there is an important flaw that can be exploited to stop MRSA. The essence of resistance, the PBP2a enzyme, does not operate single-handedly. PBP2a functionality requires wall teichoic acid, or WTA, to keep the enzyme in its proper location and teichoic acid the target for BPEI attack.
Restoring the efficacy of β-lactam antibiotics against MRSA occurs with many different compounds with many different targets. [26-30] A common theme is weakening the cell envelope framework by interrupting the cytoplasmic expression and membrane translocation of essential proteins, enzymes and precursors required for the assembly of peptidoglycan, lipoteichoic acid and WTA.
OU’s work describes an approach that significantly departs from the status quo by deactivating mature WTA in situ through electrostatic interactions with branched polyethylenimine. [14] An
example of the current state of the art is preventing the biosynthesis of anionic wall teichoic acid polymers, thereby disrupting PBP2a, restoring potency to β-lactam antibiotics and stopping the growth of MRSA. [31] Laboratory studies demonstrate this paradigm with genetic mutants that lack WTA molecules. WTA-deficient strains of MRSA are re-sensitized to amoxicillin, ampicillin, methicillin, nafcillin and ceftizoxime. [31] Thus, WTA is a potential drug target. [32]
Unfortunately, developing WTA inhibitors has been slow and the compounds have failed in pre-clinical trials. For instance, tunicamycin and ticoclopidine re-sensitize MRSA to β-lactams such as methicillin, oxacillin and cefotaxime. [31,33] These compounds, however, are unlikely to advance past pre-clinical trials. [34] Inhibition of a WTA regulatory protein with Targocil® also re-sensitizes MRSA strains to traditional β-lactams, [35-38] but this and related compounds have also failed in pre-clinical trials. [39]
OU’s methodology allows the biosynthesis of WTA to continue and uses cationic polymers, such as branched polyethylenimine, that bind to WTA and disable its function in a direct manner. [40] The overall premise is that a new antibiotic could arise from a combination of BPEI and β-lactam antibiotics (See Figure 1G). A combination treatment of BPEI and traditional and β-lactam
antibiotics is possible because these compounds are readily available, low cost and exhibit synergy against MRSA.
Targeting WTA restores β-lactam antibiotic efficacy against MRSA; BPEI potentiation of β-lactams occurs with MRSA; BPEI binds to bacterial cells in regions where WTA is located; and the anionic WTA backbone interacts with cationic BPEI. Formulations of an antibiotic with a compound that blocks the resistance pathway are a viable therapeutic strategy. For example, β-lactam antibiotics can be deactivated by bacteria that possess β-lactamases, a growing cause of resistance. [41] Clavulanic acid is a β-lactamase inhibitor that restores β-lactam efficacy. [41,42] The amoxicillin formulation is marketed as Augmentin and is now available in generic form. The success of β-lactam + β-lactamase inhibitor is an example that a combination therapy can be clinically
and commercially viable.
In the long term, OU envisions combining Augmentin® with BPEI to create a formidable antibiotic. As oxacillin is resistant to β-lactamase, [43] however, a BPEI + oxacillin combination would be complementary to an BPEI + Augmentin® combination.
The expected research contribution is the ability to provide strong evidence of the importance of BPEI + β-lactam formulations, which enables opportunities to develop new first-line antibiotics. The significance of this contribution is a reinvigoration of obsolete ineffective antibiotics. Likewise, improved first-line antibiotics will have concrete benefits of more effective treatments of infections that are drug susceptible, resistant or both that will limit tissue damage.
Achieving treatment success in a timely fashion will decrease morbidity and mortality. The patient will not have to endure multiple treatments with an array of antibiotics to clear the infection, thereby improving quality of life. [2] Shorter hospital stays and fewer surgeries to treat damaged tissue will reduce the cost of medical care. [2] If the infection is treated quickly, expensive medical diagnostic tests to confirm the presence and strain causing bacteremia will not be needed. [2]
The next stage of research involves further laboratory testing of the new antibiotic formulation to determine if this new approach is feasible for use to treat humans. While hurdles exist, the low molecular weight BPEI polymers provide guarded optimism because the polymer is very water soluble. The strong hydrophilic nature allows for IV and oral dosing, reduces protein binding
effects, limits lipophilicity and reduces cytotoxicity from membrane permeation. [44]
Data collection is underway to evaluate in vitro inhibition of MRSA growth in the presence of serum and the ability to kill different MRSA strains. Likewise, OU has been able to validate the BPEI drug binding target (WTA), its mechanism of action and mode of action.
Shortly, OU will be evaluating cytotoxicity against skin, liver and kidney cells. OU has also established a valid bioanalytical technique, high-performance liquid chromatography, to demonstrate low protein binding, in vivo measurement of free BPEI concentration in murine blood, and an in vivo estimation of BPEI half-life in murine blood. Other critical questions in drug discovery
will be examined in future work: in vivo ADME (adsorption, distribution, metabolism, excretion) and in vivo evaluation of liver toxicity, kidney toxicity and maximum tolerable dose.
Thus, while these efforts will take time, research developments at the OU are beginning the crack the armor of MRSA bacteria and bring effective treatments within sight.
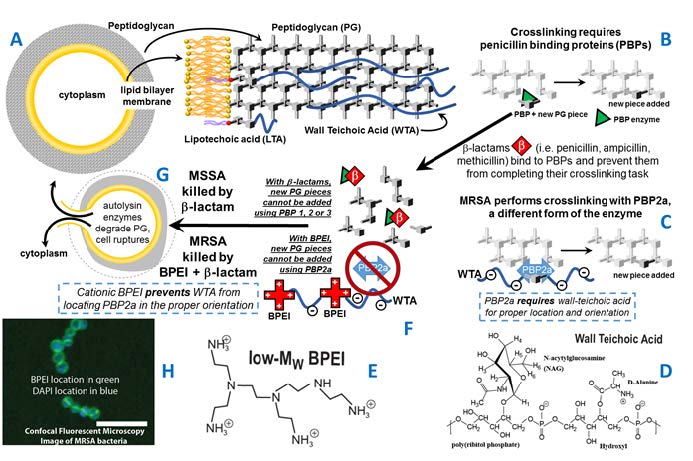
Figure 1. The cell envelope of MRSA (A) is composed of a membrane, peptidoglycan, and teichoic acids. PBP enzymes perform crosslinking (B). Most PBPs are disabled by β-lactams yet MRSA also uses PBP2a (C) for crosslinking. WTA (D) is required to locate PBP2a. Cationic polymers, such as low-MW BPEI (E), can bind to WTA (F) which prevents peptidoglycan crosslinking. The end result is a thinning of the peptidoglycan leading to cell death (G). BPEI with a fluorescent molecule provides a probe to identify the location of BPEI. Optical section of BPEI bound to MRSA (H), imaged by LSCM, stained with BPEI-AlexaFluor 488 (green) and DAPI (blue). The merged image shows BPEI binding to the cell wall and DAPI in the cytoplasm (scale bar = 5 μm).
References
1. Dantes R, Mu Y, Belflower R, Aragon D, Dumyati G, Harrison LH, Lessa FC, Lynfield R, Nadle J, Petit S, Ray SM, Schaffner W, Townes J, Fridkin S, Program-Active EI. National Burden of Invasive Methicillin-Resistant Staphylococcus aureus Infections, United States, 2011. Jama Intern Med.2013;173(21):1970-8. doi: DOI 10.1001/jamainternmed.2013.10423. PubMed PMID:
ISI:000330954300006.
2. Cosgrove SE, Qi YL, Kaye KS, Harbarth S, Karchmer AW, Carmeli Y. The impact of methicillin-resistance in Staphylococcus aureus bacteremia on patient outcomes: Mortality, length of stay, and hospital charges. Infect Cont Hosp Ep. 2005;26(2):166-74.doi: Doi 10.1086/502522. PubMed PMID: ISI:000227014000012.
3. Jain R, Kralovic SM, Evans ME, Ambrose M, Simbartl LA, Obrosky DS, Render ML, Freyberg RW, Jernigan JA, Muder RR, Miller LJ, Roselle GA. Veterans Affairs Initiative to Prevent Methicillin Resistant Staphylococcus aureus Infections. new England journal of medicine. 2011;364(15):1419.
4. Quezada Joaquin NM, Diekema DJ, Perencevich EN, Bailey G, Winokur PL, Schweizer ML. Long-term risk for readmission, methicillin-resistant Staphylococcus aureus (MRSA) infection, and death among MRSA-colonized veterans. Antimicrob Agents Chemother. 2013;57(3):1169-72. Epub 2012/12/21. doi: 10.1128/AAC.01968-12. PubMed PMID: 23254427; PMCID: 3591925.
5. Jones M, Ying J, Huttner B, Evans M, Maw M, Nielson C, Rubin MA, Greene T, Samore MH. Relationships between the importation, transmission, and nosocomial infections of methicillin-resistant Staphylococcus aureus: an observational study of 112 Veterans Affairs Medical Centers. Clin Infect Dis. 2014;58(1):32-9. Epub 2013/10/05. doi: 10.1093/cid/cit668. PubMed PMID:
24092798.
6. Nelson RE, Stevens VW, Khader K, Jones M, Samore MH, Evans ME, Douglas Scott R, 2nd, Slayton RB, Schweizer ML, Per-encevich EL, Rubin MA. Economic Analysis of Veterans Affairs Initiative to Prevent Methicillin-Resistant Staphylococcus aureus Infections. Am J Prev Med. 2016;50(5 Suppl 1):S58-65. Epub 2016/04/23. doi: 10.1016/j.amepre.2015.10.016. PubMed PMID:
27102860.
7. Hicks LA, Bartoces MG, Roberts RM, Suda KJ, Hunkler RJ, Taylor TH, Jr., Schrag SJ. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis. 2015;60(9):1308-16. doi: 10.1093/cid/civ076. PubMed PMID: 25747410.
8. Kopp BJ, Nix DE, Armstrong EP. Clinical and economic analysis of methicillin-susceptible and -resistant Staphylococcus aureus infections. Ann Pharmacother. 2004;38(9):1377-82. doi: 10.1345/aph.1E028. PubMed PMID:ISI:000223442300004.
9. Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, M JR, Talan DA, Chambers HF, Infectious Diseases Society of A. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18-55.doi: 10.1093/cid/ciq146. PubMed PMID: 21208910.
10. Gandra S, Barter DM, Laxminarayan R. Economic burden of antibiotic resistance: how much do we really know? Clin Microbiol Infect. 2014;20(10):973-80. Epub 2014/10/03. doi:10.1111/1469-0691.12798. PubMed PMID: 25273968.
11. Antonanzas F, Lozano C, Torres C. Economic Features of Antibiotic Resistance: The Case of Methicillin-Resistant Staphylococcus aureus. Pharmacoeconomics. 2015;33(4):285-325. doi: 10.1007/s40273-014-0242-y. PubMed PMID: ISI:000352276300001.
12. Leski TA, Tomasz A. Role of penicillin-binding protein 2 (PBP2) in the antibiotic susceptibility and cell wall cross-linking of Staphylococcus aureus: evidence for the cooperative functioning of PBP2, PBP4, and PBP2A. J Bacteriol. 2005;187(5):1815- 24. Epub 2005/02/18. doi: 10.1128/JB.187.5.1815-1824.2005. PubMed PMID:15716453; PMCID: 1064008.
13. Memmi G, Filipe SR, Pinho MG, Fu Z, Cheung A. Staphylococcus aureus PBP4 is essential for beta-lactam resistance in community-acquired methicillin-resistant strains. Antimicrob Agents Chemother. 2008;52(11):3955-66. Epub 2008/08/30. doi: 10.1128/AAC.00049-08. PubMed PMID:18725435; PMCID: 2573147.
14. Foxley MA, Friedline AW, Jensen JM, Nimmo SL, Scull EM, King JR, Strange S, Xiao M, Smith BE, Thomas KJI, Glatzhofer DT, Cichewicz RH, Rice CV. Efficacy of Ampicillin Against Methicillin-Resistant Staphylococcus aureus Restored Through Synergy with Branched Poly(ethylenimine). Journal of Antibiotics. 2016;Published on-line May 18. Epub May 18, 2016. doi: 10.1038/ja.2016.44; PMCID: 27189119.
15. Pastagia M, Kleinman LC, de la Cruz EGL, Jenkins SG. Predicting Risk for Death from MRSA Bacteremia. Emerg Infect Dis. 2012;18(7):1072-80. doi: 10.3201/eid1807.101371. PubMed PMID:
ISI:000306034600006.
16. Mangili A, Bica I, Snydman DR, Hamera DH.Daptomycin-resistant, methicillin-resistant Staphylococcus aureus bacteremia. Clinical Infectious Diseases. 2005;40(7):1058-60.doi: Doi 10.1086/428616. PubMed PMID: ISI:000227527100025.
17. von Eiff C, Peters G, Heilmann C. Pathogenesis of infections due to coagulasenegative staphylococci. The Lancet Infectious Diseases. 2002;2(11):677-85. doi: 10.1016/ s1473-3099(02)00438-3.
18. O’Daniel PI, Peng Z, Pi H, Testero SA, Ding D, Spink E, Leemans E, Boudreau MA, Yamaguchi T, Schroeder VA, Wolter WR, Llarrull LI, Song W, Lastochkin E, Kumarasiri M, Antunes NT, Espahbodi M, Lichtenwalter K, Suckow MA, Vakulenko S, Mobashery S, Chang M. Discovery of a New Class of Non-β-lactam Inhibitors of Penicillin-Binding Proteins with Gram-Positive Antibacterial Activity. J Am Chem Soc. 2014;136(9):3664-72. doi: 10.1021/ja500053x.
19. Zhanel GG, Love R, Adam H, Golden A, Zelenitsky S, Schweizer F, Gorityala B, Lagace-Wiens PRS, Rubinstein E, Walkty A, Gin AS, Gilmour M, Hoban DJ, Lynch JP, III, Karlowsky JA. Tedizolid: A Novel Oxazolidinone with Potent Activity Against Multidrug- Resistant Gram-Positive Pathogens. Drugs. 2015;75(3):253-70. doi: 10.1007/ s40265-015-0352-7.
20. Ling LL, Schneider T, Peoples AJ, Spoering AL, Engels I, Conlon BP, Mueller A, Schaberle TF, Hughes DE, Epstein S, Jones M, Lazarides L, Steadman VA, Cohen DR, Felix CR, Fetterman KA, Millett WP, Nitti AG, Zullo AM, Chen C, Lewis K. A new antibiotic kills pathogens without detectable resistance. Nature (London, U K). 2015;517(7535):455-9. doi: 10.1038/nature14098.
21. Bosso JA, Nappi J, Rudisill C, Wellein M, Bookstaver PB, Swindler J, Mauldin PD. Relationship between Vancomycin Trough Concentrations and Nephrotoxicity: a Prospective Multicenter Trial. Antimicrob Agents Chemother. 2011;55(12):5475-9. doi: Doi 10.1128/Aac.00168-11. PubMed PMID: ISI:000296920600008.
22. Koppula S, Ruben S, Bangash F, Szerlip HM. Pitfalls in Dosing Vancomycin. Am J Med Sci. 2015;349(2):137-9. PubMed PMID: ISI:000349353900006.
23. Bruniera FR, Ferreira FM, Saviolli LRM, Bacci MR, Feder D, Pedreira MDG, Peterlini MAS, Azzalis LA, Junqueira VBC, Fonseca FLA. The use of vancomycin with its therapeutic
and adverse effects: a review. Eur Rev Med Pharmaco. 2015;19(4):694-700. PubMed PMID: ISI:000351491900027.
24. Kurosu M, Siricilla S, Mitachi K. Advances in MRSA drug discovery: where are we and where do we need to be? Expert Opin Drug Dis. 2013;8(9):1095-116. doi: Doi 10.1517/17460441.2013.807246. PubMed PMID: ISI:000323502200005.
25. Wilke MH. Multiresistant Bacteria and Current Therapy – the Economical Side of the Story. Eur J Med Res. 2010;15(12):571-6. PubMed PMID: ISI:000285697900008.
26. Therien AG, Huber JL, Wilson KE, Beaulieu P, Caron A, Claveau D, Deschamps K, Donald RG, Galgoci AM, Gallant M, Gu X, Kevin NJ, Lafleur J, Leavitt PS, Lebeau-Jacob C, Lee SS, Lin MM, Michels AA, Ogawa AM, Painter RE, Parish CA, Park YW, Benton-Perdomo L, Petcu M, Phillips JW, Powles MA, Skorey KI, Tam J, Tan CM, Young K, Wong S, Waddell ST, Miesel L. Broadening the spectrum of beta-lactam antibiotics through inhibition of signal peptidase type I. Antimicrob Agents Chemother. 2012;56(9):4662-70. doi: 10.1128/AAC.00726-12. PubMed PMID: 22710113;
PMCID: PMC3421906.
27. Tan CM, Therien AG, Lu J, Lee SH, Caron A, Gill CJ, Lebeau-Jacob C, Benton-Perdomo L, Monteiro JM, Pereira PM, Elsen NL, Wu J, Deschamps K, Petcu M, Wong S, Daigneault E, Kramer S, Liang LZ, Maxwell E, Claveau D, Vaillancourt J, Skorey K, Tam J, Wang H, Meredith TC, Sillaots S, Wang-Jarantow L, Ramtohul Y, Langlois E, Landry F, Reid JC, Parthasarathy G, Sharma S, Baryshnikova A, Lumb KJ, Pinho MG, Soisson SM, Roemer T. Restoring Methicillin-Resistant Staphylococcus aureus Susceptibility to beta-Lactam Antibiotics. Sci Transl Med. 2012;4(126). PubMed PMID:WOS:000302129100004.
28. Xia GQ, Kohler T, Peschel A. The wall teichoic acid and lipoteichoic acid polymers of Staphylococcus aureus. Int J Med Microbiol. 2010;300(2-3):148-54. doi: 10.1016/j. ijmm.2009.10.001. PubMed PMID: WOS:000274701400011.
29. Pinho MG, de Lencastre H, Tomasz A. An acquired and a native penicillin-binding protein cooperate in building the cell wall of drug-resistant staphylococci. P Natl Acad Sci USA. 2001;98(19):10886-91. doi: DOI 10.1073/pnas.191260798. PubMed PMID: WOS:000170966800070.
30. Komatsuzawa H, Suzuki J, Sugai M, Miyake Y, Suginaka H. Effect of Combination of Oxacillin and Non-Beta-Lactam Antibiotics on Methicillin-Resistant Staphylococcus-Aureas. J Antimicrob
Chemoth. 1994;33(6):1155-63. doi: DOI 10.1093/jac/33.6.1155. PubMed PMID: WOS:A1994NT76900008.
31. Farha MA, Leung A, Sewell EW, D’Elia MA, Allison SE, Ejim L, Pereira PM, Pinho MG, Wright GD, Brown ED. Inhibition of WTA Synthesis Blocks the Cooperative Action of PBPs and Sensitizes MRSA to β-Lactams. ACS Chem Biol. 2013;8(1):226-33. doi: 10.1021/cb300413m.
32. Pasquina LW, Santa Maria JP, Walker S. Teichoic acid biosynthesis as an antibiotic target. Curr Opin Microbiol. 2013;16(5):531- 7. doi: 10.1016/j.mib.2013.06.014.
33. Campbell J, Singh AK, Maria JPS, Kim Y, Brown S, Swoboda JG, Mylonakis E, Wilkinson BJ, Walker S. Synthetic Lethal Compound Combinations Reveal a Fundamental Connection between Wall Teichoic Acid and Peptidoglycan Biosyntheses in Staphylococcus aureus. Acs Chem Biol. 2011;6(1):106-16. doi: Doi 10.1021/Cb100269f. PubMed PMID: ISI:000286306000012.
34. Roemer T, Schneider T, Pinho MG. Auxiliary factors: a chink in the armor of MRSA resistance to beta-lactam antibiotics. Curr Opin Microbiol. 2013;16(5):538-48. doi: DOI 10.1016/j.mib.2013.06.012. PubMed PMID: ISI:000327923800004.
35. D’Elia MA, Millar KE, Beveridge TJ, Brown ED. Wall teichoic acid polymers are dispensable for cell viability in Bacillus subtilis. J Bacteriol. 2006;188(23):8313-6. doi: Doi 10.1128/Jb.01336-06. PubMed PMID:ISI:000242275600039.
36. Bhavsar AP, Erdman LK, Schertzer JW, Brown ED. Teichoic acid is an essential polymer in Bacillus subtilis that is functionally distinct from teichuronic acid. J Bacteriol. 2004;186(23):7865-73. doi: Doi 10.1128/Jb.186.23.7865-7873.2004. PubMed PMID:ISI:000225271700005.
37. Swoboda JG, Meredith TC, Campbell J, Brown S, Suzuki T, Bollenbach T, Malhowski AJ, Kishony R, Gilmore MS, Walker S. Discovery of a Small Molecule that Blocks WallTeichoic Acid Biosynthesis in Staphylococcus aureus. Acs Chem Biol. 2009;4(10):875-83. doi: Doi 10.1021/Cb900151k. PubMedPMID: ISI:000272562000008.
38. Wang H, Gill CJ, Lee SH, Mann P, Zuck P, Meredith TC, Murgolo N, She X, Kales S, Liang L, Liu J, Wu J, Santa Maria J, Su J, Pan J, Hailey J, McGuinness D, Tan CM, Flattery A, Walker S, Black T, Roemer T. Discovery of Wall Teichoic Acid Inhibitors as Potential Anti-MRSA β-Lactam Combination Agents. Chem Biol (Oxford, U K). 2013;20(2):272- 84. doi:10.1016/j.chembiol.2012.11.013.
39. Lee SH, Wang H, Labroli M, Koseoglu S, Zuck P, Mayhood T, Gill C, Mann P, Sher X, Ha S, Yang SW, Mandal M, Yang C, Liang LZ, Tan Z, Tawa P, Hou Y, Kuvelkar R, De- Vito K, Wen XJ, Xiao J, Batchlett M, Balibar CJ, Liu J, Xiao JY, Murgolo N, Garlisi CG, Sheth PR, Flattery A, Su J, Tan C, Roemer T. TarO-specific inhibitors of wall teichoic acid biosynthesis restore beta-lactam efficacy against methicillin-resistant staphylococci. Sci Transl Med. 2016;8(329). PubMed PMID: ISI:000372079400004.
40. Foxley MA, Friedline AW, Jensen JM, Nimmo SL, Scull EM, King JR, Strange S, Xiao M, Smith BE, Thomas KJI, Glatzhofer DT, Cichewicz RH, Rice CV. Efficacy of Ampicillin Against Methicillin-Resistant Staphylo-coccus aureus Restored Through Synergy with Branched Poly(ethylenimine). Journal of Antibiotics. 2016;Accepted for Publication.
41. Geddes AM, Klugman KP, Rolinson GN. Introduction: historical perspective and development of amoxicillin/clavulanate. Int J Antimicrob Ag. 2007;30:S109-S12. PubMedPMID: ISI:000251938700001.
42. Gill EE, Franco OL, Hancock REW. Antibiotic Adjuvants: Diverse Strategies for Controlling Drug-Resistant Pathogens. Chem Biol Drug Des. 2015;85(1):56-78. PubMed PMID: ISI:000346498500007.
43. Graninger W, Wenisch C, Hasenhundl M. Treatment of Staphylococcal Infections. Curr Opin Infect Dis. 1995;8:S20-S8. PubMed PMID: WOS:A1995QQ38400005.
44. Gualtieri M, Baneres-Roquet F, Villain-Guillot P, Pugniere M, Leonetti JP. The Antibiotics in the Chemical Space. Curr Med Chem. 2009;16(3):390-3. PubMed PMID: WOS:000263294800010.