The advent of wearable or body-borne electronics is rapidly changing how the Department of Defense (DoD) provides diagnostic and therapeutic medical care to the warfighter [1].
Multiple DoD entities, from the U.S. Army Combat Capabilities Development Command’s Chemical Biological Center, to the Defense Threat Reduction Agency [1], are seeking bioelectronics that can transform military medicine by providing medics with valuable information to improve acute care on the battlefield, and aiding military doctors providing prolonged care [2]. For instance, bioelectronics sensors that measure multiple signals, including heartbeat and the secretion of metabolites in perspiration [3], can provide remote monitoring of warfighter medical status during operations. Next-generation bioelectronics can be delivered by implantation [4, 5] or can be swallowed [6, 7] so as to deliver therapeutic medications.
A seamless integration of such bioelectronics with the soft, complex, and 3D shape of the human body is inherently challenging due to the geometrical, material, and mechanical dichotomies between the two. Conventional electronics are typically fabricated via planar, top-down processes on a rigid substrate. Conversely, the human body is an irregularly shaped and highly flexible, stretchable construct [5]. Significant research has been dedicated to overcoming this challenge, including the design of stretchable, flexible electronics, the development of electronic skin tattoos, and the manufacturing of electronic textile and bioelectronic implants [3, 5, 8]
This article proposes and highlights the advancement of a multimaterial and multiscale 3D printing approach that can enable the fabrication of bioelectronics to better interface with the human body. Specifically, the article highlights the development of (a) a freeform electronics fabrication approach that allows for the creation of complex 3D systems [9, 10], and (b) the multimaterial-printing of an ingestible gastric resident system that allows for non-surgical and needle-free delivery of wireless electronics into the human body [7, 11].

Figure 1. Multi-scale 3D printing of functional devices and bioelectronics [5]. Reproduced with permission. Copyright 2016 Elsevier.
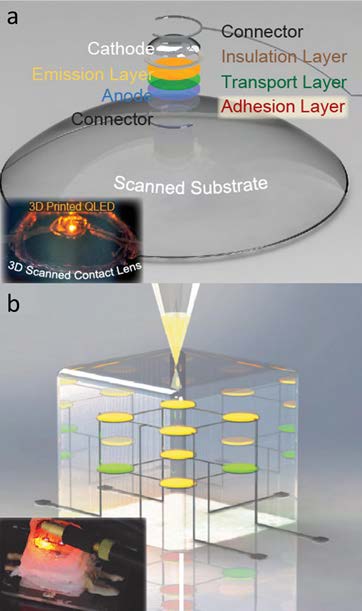
Figure 2. (a) 3D printed QD-LED on a 3D-scanned contact lens onto a curvilinear substrate. The inset shows the electroluminescence output from the printed QD-LED on a 3D scanned contact lens [12]. (b) 3D printed 2 × 2 × 2 multidimensional array of embedded QD-LEDs. The inset shows the electroluminescence output from the printed QD-LEDs within a 3D matrix [12]. Reproduced with permission. Copyright 2014 American Chemical Society
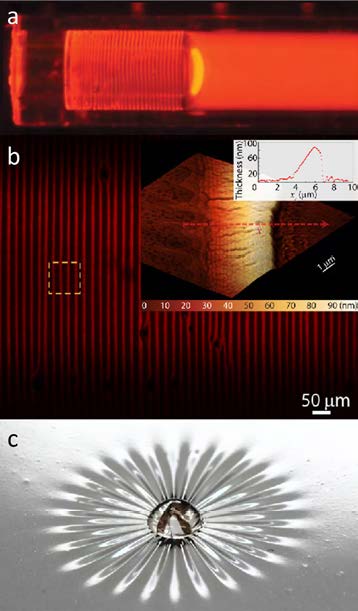
Figure 3. (a) QDs bands microstructure formed inside a capillary tube during a drying process. Fluorescence image of bands formed from the QDs as toluene evaporates from the open end on the left [18]. (b) Confocal microscopy image showing QD band arrays along the capillary tubes. Larger inset shows the Atomic Force Microscopy image of one of the bands. Smaller inset shows the profilometry measurement extracted along the path of the red arrow on the AFM image [18]. (c) Photograph of the deposition of silica nanoparticles on an elastomeric membrane to study the drying-induced stress by characterizing the wrinkles formation. Figure 3a and 3b reproduced with permission. Copyright 2015 American Chemical Society
Multiscale 3D Printing of Electronic Devices
3D printing is a broad class of manufacturing technologies, first invented in the 1980s. However, the majority of 3D printing capabilities remain somewhat limited to the use of specific plastics, passive conductors, and certain biological materials. A multiscale, extrusion-based 3D printing approach can enable the integration of diverse classes of materials to create a variety of electronics and functional devices not easily achieved using standard microfabrication techniques [5, 9, 10, 12–16]. The ability to 3D print various classes of materials possessing distinct properties could enable the freeform generation of active electronics in unique, functional, interwoven architectures. For example, functional nanomaterials can be dispersed in solvents to form solution-processable inks, which can be integrated into micron-scale coating or printing processes, as illustrated in Figure 1.
Using a 3D-scanned topology and direct fabrication approach, electronics can be manufactured without complicated device design or transfer procedures. As a proof of concept, Figure 2a depicts a quantum dot light-emitting diode (QD-LED) on a 3D-scanned contact lens, and Figure 2b shows a 2×2×2 array of multicolored QD-LEDs embedded within a silicone cube. Both structures were created using a direct printing approach [12]
The multiscale 3D printing approach leverages the evaporative-assembly of functional nanomaterials during printing, which allows the sole execution of the deposition process with a desktop-sized microextrusion based printer. This can enable an easily deployable electronics 3D printing strategy, whereby small, portable printers can directly print highly customized functional devices, such as smart bandages, onto skin at the location needed.
The ability to govern the deposition of nanomaterials dictates the performance of advanced printed devices. However, nanomaterials typically do not spontaneously form a uniform and thin film during deposition. Instead, the process often results in a wide range of interesting features, such as the so-called “coffee rings” where most nanoparticles will be concentrated on the edge of a pinned contact line.
A variety of platforms have been proposed to better understand and ultimately control these dynamic and complex forces [17, 18]. For example, multiscale characterization of nanomaterials deposited in a confined construct can help elucidate the complex relationship between drying parameters (such as evaporation speed) and microstructure morphologies (see Figure 3a and Figure 3b) [18]. In another example, the drying-induced stress of a colloid can be characterized in real time by studying the formation of wrinkles that appear as a colloidal suspension deposited on a thin elastic membrane dries (see Figure 3c) [17].
Multimaterial 3D Printing of Ingestible Gastric Resident Electronics
As described above, multiscale 3D printing provides an opportunity to create biocompatible medical devices that can be used in regions that cannot be accessed by wearable, textile-based or epidermal electronics [8]. Nevertheless, continued research and development is needed to overcome the remaining challenges. Furthermore, direct implantation often requires surgical processes, and most surgically-placed medical implants carry an inherent risk of eliciting foreign body immune system responses [4]. Implanted devices can serve as a nidus for infection, which can require immediate operative intervention that is unavailable in remote locations.
Advancements in ingestible electronics can allow for the oral delivery of devices directly into the body, enabling a non-surgical, needle-free approach that obviates concerns of adverse immune responses or infection [6]. Importantly, unlike most other organs, the stomach is a relatively immune privileged site in the human body; this enables the long-residence of devices without causing an immune response. However, despite decades of interest and research since its invention in the 1950s, ingestible electronics have yet to acquire the capability to reside in the hostile and dynamic gastric environment for a duration beyond just a few days [19, 20].
Multi-material 3D printing can enable the creation of a wide range of multifunctional, robust, and transformable architecture [16]. For example, this enables the creation of “gastric resident architecture” (GRE) [7, 11], which is a structure that can be folded into the shape of a capsule, and upon ingestion and exposure to gastric fluid in the gastric space, expands into a mechanical configuration that prolongs its residence (see Figure 4a–e).
For the first time, this has enabled the gastric residence of electronics up to a month in duration, before disintegration of multimaterial interfaces that allow the excretion of the device. Indeed, the integration with various active electronics, wireless module and drug delivery components (see Figures 4f and 4g) via multi-material 3D printing approaches can ultimately enable seamless interfaces with both personal electronics and the delivery of therapeutic agents, allowing a next-generation digital diagnosis and remote interventions.
Figure 5a shows a computer-aided design model of the GRE device highlighting various key components, such as the gastric resident architecture, Bluetooth wireless-microcontroller and antenna, power system (batteries) for communications and control, and personalized drug delivery modules. The 3D-printed GRE device (see Figure 5b) can be folded into a capsule-size dosage form for oral delivery, as shown in the deployed device in a porcine stomach (see Figure 5c).
We also show the GRE’s ability to perform long-term wireless bilateral communications and control of the ingested device with a personal device via Bluetooth connection. We acquired long-resident, real-time core temperature data directly from a GRE wirelessly transmitting to an Android device for up to 15 days. The receiver devices do not require an additional adaptor or communication device to function (see Figure 6).
The connection strength is limited to the distance of an arm’s length (<100 cm) to provide physical signal isolation. In future work, various gastric-tolerant drug delivery architectures (such as with a gold membrane) can be integrated to enable on-demand or programmable delivery of therapeutic agents [21].
Future works can also leverage the development of wireless powering and energy harvesting strategies, which can further extend the electronic functionalities in the dynamic and hostile gastric environment beyond a month. We envision that the freeform fabrication of ingestible wireless electronics from a desktop-sized 3D printer can enable a next-generation remote monitoring, diagnosis, and treatment platform for the warfighter.
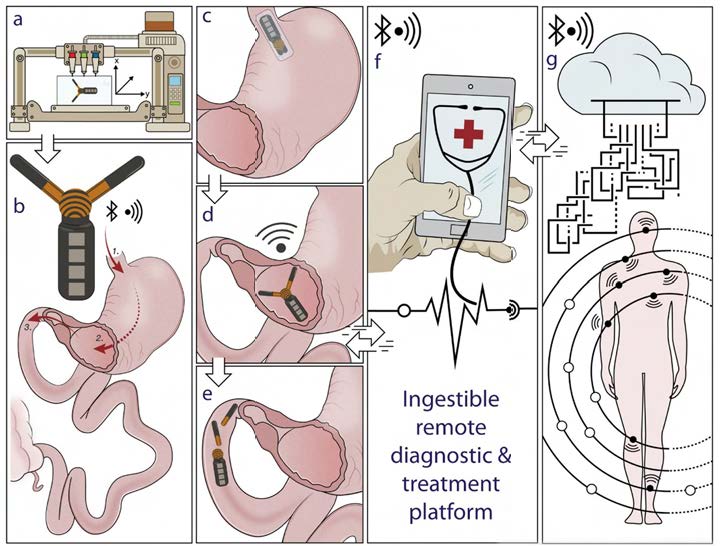
Figure 4. Illustration of 3D-printed gastric resident electronics (GRE) concept. (a–e): a desktop-sized multimaterial 3D printer can be used to fabricate a highly customized GRE which to be delivered orally, reside in the stomach for weeks, and finally break up to be excreted from the gastric space. (f–g): GRE can form bilateral communications with personal devices suchas a smart phone directly for communications and controls. The seamless interconnection with other wireless wearable electronics and implants enables a real-time feedback-based automated diagnostic and responsive treatment. [7] Reproduced from Reference [7] under the terms of the Creative Commons Attribution License (CC BY). Copyright 2018 John Wiley and Sons.

Figure 5. (a) 3D models of GRE device components, such as the gastric resident architecture, personalized drug delivery modules, electronics and power system for communications and control. (b) Optical photograph of the 3D printed GRE. (c) X-ray image of the deployed GRE in a porcine stomach. [7] Reproduced from Ref. [7] under the terms of the Creative Commons Attribution License (CC BY). Copyright 2018 John Wiley and Sons
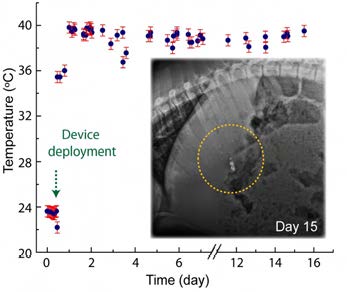
Figure 6. The in vivo result demonstrating the ability to achieve bilateral communications and control of GRE. A direct, real-time core-temperature measurement has been performed with a tablet attached on the wall of the cage over 17 days. Inset shows the X-ray images of the GRE on day 15, highlighting the robustness of the GRE to remain functional in the dynamic and hostile gastric environment for weeks. [7] Reproduced from Ref. [7] under the terms of the Creative Commons Attribution License (CC BY). Copyright 2018 John Wiley and Sons.
Conclusion
This multiscale, multi-material 3D printing electronics fabrication approach can enable the fabrication of bioelectronics that can better interface and integrate with the warfighter, and can realize a transformative remote diagnostic, monitoring, and treatment strategy. For example, this enables the freeform 3D fabrication of unique hybrid bioelectronics that cannot be created through conventional fabrication. We envision this approach can transform military medicine by enabling the seamless integration of electronics with the human body—ultimately enhancing the safety and well-being of service members before, during, and after training and operations.
Acknowledgements
The author acknowledges funding support in part from the Department of Mechanical Engineering, University of Utah, as well as the National Science Foundation under Emerging Frontiers in Research and Innovation (EFRI) Program (Grant No. EFRI 1830958). The author thanks Joel
Hewett and Dr. Yu Kee Ooi for their feedback.
References
1. Hirschberg, D., Betts, K., & Emanuel, P. (2014). Assessment of Wearable Sensor Technologies for Biosurveillance. Belcamp, MD.: U.S. Army Research, Development and Engineering Command, Edgewood Chemical Biological Center. Retrieved from http://www.dtic.mil/dtic/tr/fulltext/u2/a611718.pdf
2. Keenan, S., & Riesberg, J. (2017). Prolonged Field Care: Beyond the “Golden Hour”. Wilderness & Environmental Medicine, 28(2), S135-S139. doi: 10.1016/j.wem.2017.02.001
3. Gao, W., Emaminejad, S., Nyein, H., Challa, S., Chen, K., & Peck, A., … & Javey, A. (2016). Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature, 529(7587), 509-514. doi: 10.1038/nature16521
4. Farra, R., Sheppard, N., McCabe, L., Neer, R., Anderson, J., & Santini, J., … & Langer, R. (2012). First-in-Human Testing of a Wirelessly Controlled Drug Delivery Microchip. Science Translational Medicine, 4(122), 122ra21-122ra21. doi: 10.1126/scitranslmed.3003276
5. Kong, Y. L., Gupta, M., Johnson, B., & McAlpine, M. (2016). 3D printed bionic nanodevices. Nano Today, 11(3), 330-350. doi: 10.1016/j.nantod.2016.04.007
6. Awasthi, R., & Kulkarni, G. (2014). Decades of research in drug targeting to the upper gastrointestinal tract using gastroretention technologies: where do we stand?. Drug Delivery, 23(2), 378-394. doi: 10.3109/10717544.2014.936535
7. Kong, Y. L., Zou, X., McCandler, C., Kirtane, A., Ning, S., & Zhou, J., … & Traverso, G. (2018). 3D-Printed Gastric Resident Electronics. Advanced Materials Technologies, 1800490. doi: 10.1002/admt.201800490
8. Ghosh, U., Ning, S., Wang, Y., & Kong, Y. L. (2018). Addressing Unmet Clinical Needs with 3D Printing Technologies. Advanced Healthcare Materials, 7(17), 1800417. doi: 10.1002/adhm.201800417
9. M. C. McAlpine and Y. L. Kong, “3D printed active electronic materials and devices,” US Patent 9,887,356, issued February 6 2018.
10. M. C. McAlpine, M. Sebastian-Mannoor, Y. L. Kong, B. N. Johnson. “Multi-functional hybrid devices/ structures using 3D printing.” U.S. Patent 9,517,128 issued December 13, 2016.
11. R. Langer, G. Traverso, and Y. L. Kong, “Gastric resident electronics.” U.S. Patent Application 16/202,647.”
12. Kong, Y. L., Tamargo, I., Kim, H., Johnson, B., Gupta, M., & Koh, T., … & McAlpine, M. (2014). 3D Printed Quantum Dot Light-Emitting Diodes. Nano Letters, 14(12), 7017- 7023. doi: 10.1021/nl5033292
13. Gupta, M., Meng, F., Johnson, B., Kong, Y. L., Tian, L., & Yeh, Y., … & McAlpine, M. (2015). 3D Printed Programmable Release Capsules. Nano Letters, 15(8), 5321-5329. doi: 10.1021/acs.nanolett.5b01688
14. Johnson, B., Lancaster, K., Hogue, I., Meng, F., Kong, Y. L., Enquist, L., & McAlpine, M. (2016). 3D printed nervous system on a chip. Lab On A Chip, 16(8), 1393-1400. doi: 10.1039/c5lc01270h
15. Johnson, B., Lancaster, K., Zhen, G., He, J., Gupta, M., & Kong, Y. L., … & McAlpine, M. (2015). 3D Printed Anatomical Nerve Regeneration Pathways. Advanced Functional Materials, 25(39), 6205-6217. doi: 10.1002/ adfm.201501760
16. Mannoor, M., Jiang, Z., James, T., Kong, Y. L., Malatesta, K., & Soboyejo, W., … & McAlpine, M. (2013). 3D Printed Bionic Ears. Nano Letters, 13(6), 2634-2639. doi: 10.1021/nl4007744
17. Boulogne, F., Kong, Y. L., Nunes, J., & Stone, H. (2016). Effect of the Polydispersity of a Colloidal Drop on Drying Induced Stress as Measured by the Buckling of a Floating Sheet. Physical Review Letters, 116(23). doi: 10.1103/physrevlett.116.238001
18. Kong, Y. L., Boulogne, F., Kim, H., Nunes, J., Feng, J., & Stone, H. (2015). Deposition of Quantum Dots in a Capillary Tube. Langmuir, 31(45), 12560-12566. doi: 10.1021/acs.langmuir.5b03443
19. Bettinger, C. (2018). Advances in Materials and Structures for Ingestible Electromechanical Medical Devices. Angewandte Chemie International Edition, 57(52), 16946- 16958. doi: 10.1002/anie.201806470
20. Bettinger, C. (2015). Materials Advances for Next-Generation Ingestible Electronic Medical Devices. Trends In Biotechnology, 33(10), 575-585. doi: 10.1016/j.tibtech.2015.07.008
21. Nadeau, P., El-Damak, D., Glettig, D., Kong, Y. L., Mo, S., & Cleveland, C., … & Traverso, G. (2017). Prolonged energy harvesting for ingestible devices. Nature Biomedical Engineering, 1(3), 0022. doi: 10.1038/s41551-016-0022


