Introduction
Two-dimensional nanomaterials, such as graphene and transition metal dichalcogenides, have tremendous potential to broaden the range of materials used by the Department of Defense. In particular, they are very useful in electrical energy storage applications.Due to their unique layered structures and high electronic conductivities, 2D nanomaterials can employ effective and efficient methods of storing energy [1]. Energy storage has erupted as one of the world’s greatest challenges, resulting in the stimulation of research to push the limits of materials and technology. From medium (vehicles) to small (personal electronics) dimensions, devices such as batteries and electrochemical capacitors store energy electrochemically. Devices that can perform in extreme environments, provide high energy and power, and can be supplied in configurations that are flexible and conformable have become desirable, as current U.S. DoD initiatives strive to improve upon these characteristics with novel 2D materials. Two-dimensional materials can be fabricated into electrodes, which can be morphed into a variety of shapes and even integrated into structures or garments to create multifunctional materials. The horizon is wide for 2D materials, as they have the potential to be useful in many functional applications, but are currently investigated by how they influence the performance of conventional energy storage devices such as secondary batteries and electrochemical capacitors[2] .
In 2011, material scientists at Drexel University discovered an expansive family of 2D transition metal carbides, carbonitrides and nitrides named MXenes (M – transition metal, X – carbon or nitrogen) [3]. The name MXene originated from the structure resembling other 2D materials, such as graphene. Thus, new horizons opened up in the 2D world. This article focuses on energy storage applications of this new family of materials, but synthesis of MXenes, manufacturing of their composites and related structures, as well as a variety of other potential applications will be described.
The MXenes were produced by the removal, or selective extraction, of specific ‘A’ elements, such as aluminum or gallium, from their MAX precursors (see Figure 1) [3,4]. The MAX phases are a family of ternary metal carbides and/or nitrides, listed in Table 1, where they are separated into three classes. They exhibit a formula of Mn+1AXn, where the ‘M’ element is typically an early transition metal (e.g., Ti, Nb, V, Mo, Ta, etc.), the ‘A’ element is mostly IIIA and IVA group members (e.g., Al, Si, Ga, etc.), and X is carbon and/or nitrogen [5].
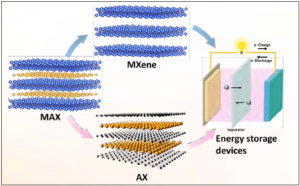
Figure 1. Image shows the production of MX (MXene) and AX materials by selective extraction of ‘A’ or ‘M’ element from the precursor MAX phase. Both phases can be used as active materials in energy storage devices, such as batteries and electrochemical capacitors. (Released)
Table 1. Some of the MAX phases reported, organized by MX structure [9]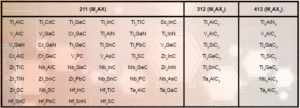
After the removal of ‘A’ layers from a MAX phase by etching in an acid, the resulting material has a crystalline 2D MX structure (carbide, nitride or carbonitride), thus MXene [3,6,7]. As a result of wet chemical etching, the surface of MXene is terminated by oxygen or fluorine containing groups, therefore, the complete formula is written as Mn+1XnTx, where ‘T’ stands for surface termination [6]. This surface chemistry dictates many properties of MXenes, in particular making them hydrophilic. If the ‘M’ layer is removed, AX structures can be produced, but the layers become amorphous/disordered (see Figure 1) [8].
MXenes exhibit a 2D structure with subnanometer thick layers, similar to graphene. The major difference between graphene and MXene is compositional: graphene is composed of a network of carbon atoms and MXenes are 2D compounds built of metal and nonmetal ions, like transition metal dichalcogenides or oxides. The majority of MXenes produced to date (about 20) are carbides and are focused on in this article. They come in three different structures (M2CTx, M3C2Tx and M4C3Tx) [6]. This structural diversity enables MXenes to become materials-by-design, thus facilitating multiple configurations, properties and applications, similar to transition metal dichalcogenides, but with a larger diversity and different properties.
MXenes, such as Ti3C2Tx, offer high electronic conductivities up to 7000 S/cm [10], comparable to metals. They are hard, strong, wear resistant, thermally stable in inert environments and have a strong affinity for water (hydrophilic). Because of their high conductivities and layered structures, MXenes have primarily been studied as active materials in energy applications [6]. The MXenes exhibit superior capacity for the reversible intercalation of most metal cations, including Li+, Na+, K+ and multivalent ions, such as Mg2+, and Al3+ and a variety of organic molecules [11,12]. This reversible process offers mechanisms for charge storage across various technologies, shedding light on the possible use of MXenes as electrode materials for lithium-ion and alternative energy storage systems [11]. For example, magnesium and aluminum ions were shown to intercalate MXenes from aqueous solutions providing the basis for future Mg- and Al-ion battery applications. [11]
MXene Synthesis, Delamination and Integration with Other Materials
Several synthesis techniques have been used to produce MXenes and give versatility to their manufacturing. Initially, MXenes were produced by using hydrofluoric acid aqueous solution to selectively extract elemental ‘A’ layers from their corresponding MAX precursors [3]. Later on, it was found that MXenes produced by etching MAX phase in a solution of dilute hydrochloric acid and lithium fluoride exhibited a clay-like behavior due to intercalation of lithium ions during etching [13]. This method is safer than the HF method, and materials produced by the HCl/LiF etching method are both highly conductive and can easily be molded into a variety of shapes and sizes. Within several minutes, flexible MXene films can be rolled to any thickness while retaining high conductivity and arranged to be used as electrodes in energy storage devices, or conform to any shape. This rolling process looks a bit like rolling out cookie dough, with results that are even sweeter from an energy storage standpoint.
As noted previously, the layered structure of MXene is advantageous because it allows ions to enter during synthesis. This alone may be sufficient for delaminating MXenes, such as Ti3C2Tx clay, and produce colloidal solutions of single- or few-layer flakes [13]. In addition, after synthesis, the MXene layers can be separated by a variety of organic molecules, such as urea, hydrazine, dimethyl sulfoxide and tertiary amines etc., which open up the layers [12,14]. In the case of dimethyl sulfoxide or tertiary amines, these layers can be separated (delaminated) into single-layer flakes with agitation by sonication (see Figure 2). The delaminated MXenes are usually dispersed in water and can be used to prepare supported or freestanding and flexible films/papers via filtration, spray coating, spin coating or other methods allowing for largescale production [10,13,15]. These flexible MXene papers can serve directly as electrodes without current collectors due to high metallic conductivity, and exhibit improved electrochemical performance compared with those made of multilayer powders, indicating a great potential for using MXenes in flexible and wearable energy storage devices [16,17], such as combat gear, soldier-borne electronics and applications which require ruggedness, adaptability and reliability.
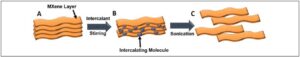
Figure 2. Schematic representing the intercalation and delamination of MXenes. A) Original multi-layer MXene, small spacing between stacked sheets; B) After the addition of an intercalating molecules and stirring, the molecules enter between the MXene sheets; C) After washing and sonication, the MXene sheets become separated and free-standing, forming a colloidal solution. (Released)
Similar to other 2D materials such as graphene, MXenes can be used as building blocks to integrate with other materials and produce MXene-based nanocomposites. For example, MXenes were used as nanofillers in a polyvinyl alcohol polymer matrix. The as-fabricated MXene/PVA nanocomposites exhibited much improved mechanical properties relative to pure PVA [18]. Additionally, small polymer chains and carbon nanomaterials (e.g., carbon nanotubes and graphene) were intercalated between MXene layers by a mixing and subsequent filtration method, resulting in MXene-based composite papers with more open structures that facilitated the diffusion of electrolyte ions [19]. The understanding of how multiple nanomaterials are able to work together cohesively allows researchers to develop and engineer hybrid materials with outstanding properties. For example, a Ti3C2Tx MXene film was tested mechanically and was able to support about 4,000 times its own weight. When made as a composite with a polymer, PVA, the film easily supported nearly 15,000 times its own weight [18].
Electrochemical Energy Storage Applications
Batteries and supercapacitors are two of the most common electrochemical energy storage devices. Batteries, which power our cell phones, computers, cars and more, store charge based on electrochemical reactions occurring within the cell. Electrochemical capacitors, also termed supercapacitors or ultracapacitors, have a different charging mechanism which relies on surface ion adsorption/desorption or redox reaction of the electrode active material (see Figure 3, left)[20]. These electrostatic interactions produce quick charging and discharging, making electrochemical capacitors well suited for power applications. Batteries and electrochemical capacitors consist of electrodes, current collectors, a separator and an electrolyte. The two electrodes, a cathode and an anode, are backed by current collectors and divided by a separator. Ionic current flows through the cell when a potential is applied, from electrode to electrode, through the electrolyte, which can be liquid, solid or gel in state. Aqueous, organic or ionic liquid electrolytes are generally used, with the organic ones used in majority of commercial energy storage devices. Each component of the device must be compatible with other components as well as exhibit properties that are associated with the specific target application.
Lithium-ion Batteries
With lithium-ion battery technology being one of the most important energy storage technologies used in defense applications [21], expanding the field to include materials that could improve or replace current ones, providing enhanced performance, has become important for many defense pursuits such as hybrid automobiles, nanoscale electronics and battlefield equipment. Two-dimensional materials, like graphene and MXene, are perfectly suited for applications as the electrode material due to their increased surface area relative to a bulk structure. This allows for larger quantities of ions to be inserted (see Figure 3, right) during charging and removed during discharging, thus increasing the amount of energy stored. Both experimental and theoretical studies on employing MXenes as anode materials for lithium-ion batteries have been conducted due to reversible insertion of Li+ between layers of the material. Using MXene in lithium-ion technology was first tested in 2012 by analyzing Ti2CTx, the lightest MXene, as the active material. It was shown that a capacity of 225 mAh/g and 70 mAh/g was achieved at C/25 and 10 C charging rates, respectively [22]. After promising results, different variations of MXenes were tested and a high capacity of approximately 600 mAh/g was yielded by Nb2CTx MXene, with excellent cycling stability [14]. Another recent study on Ti3C2Tx MXene infiltrated with tin (Sn4+) ions pushed the specific capacity to approximately 800 mAh/g at a current density of 50 mA/g, giving further improvement to MXene anodes [23]. Additionally, the value of approximately 1200 mAh/g was reported very recently for porous Ti3C2Tx-based MXene [24].
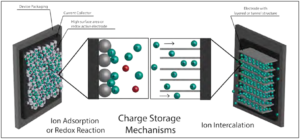
Figure 3. Schematic describing the main differences between two charge storage mechanisms: ion adsorption or pseudocapacitive redox reaction (left) and ion intercalation (right). (Released)
Supercapacitors
While lithium-ion batteries supply sufficient energy for many defense and personal pursuits, the challenges include low power density, yielding several hour charge times. On the contrary, supercapacitors challenge battery technology by allowing for millisecond-second charging times due to the fast surface or redox active electrodes. These charging mechanisms render the importance of active material performance which can provide defense applications, such as fork lifts, wind mills, solar panels, hybrid cars and battlefield equipment, with high power. MXene lends itself to be an outstanding supercapacitor not only because of the high electrical conductivity, but also due to spontaneous chemical intercalation of various cations from the electrolyte in between MXene layers. Delaminating layers of MXene increased performance in supercapacitor applications due to the 2D nature revealed after exfoliation. Reversible capacities of more than 330 F/cm3 were achieved, and more than 10,000 charge-discharge cycles were reached. Attained by Ti3C2Tx paper in an aqueous electrolyte, these performances exceeded that of the best all-carbon supercapacitors [12]. Additionally, rolled films from Ti3C2Tx clay showed even higher capacities up to 900 F/cm3, along with excellent cyclability and rate performance [11]. It was also found that hybridization of MXenes with other nanomaterials, such as polymers, nanocarbons and metal oxides, led to an improved electrochemical performance compared with pure MXenes [18,19]. MXene with a gel-based electrolyte produced films, which showed impressive capacitive performance [18]. Using electrochemically active polymer, polypyrrole, capacitive performance of the as-fabricated MXene/polymer composites can be pushed further, with a stable volumetric capacitance of approximately 1000 F/cm3 up to 25,000 cycles [25].
This work provided insight into MXene materials being used as structural energy storage devices such as actuators, sensors and electromagnetic shielding. When MXene is combined with other carbon nanomaterials, such as carbon nanotubes and graphene, performance is also increased due to the opened MXene layered structure, allowing for easy accessibility of ions, thus faster rate performance and high volumetric capacitance [19].
Hybrid Devices and Alternative-ion Batteries
Current technology offers batteries with high energy densities and capacitors with reliable power densities and a much longer lifetime. Hybrid devices, which encompass a mixture of charging mechanisms, aim to advantageously incorporate the two technologies, increasing the energy and power densities. The research team at the University of Tokyo demonstrated that Ti2CTx MXene can serve as negative electrode for high-power sodium-ion hybrid capacitors, which delivered high capacities at quite high current density (90 and 40 mAh/g at 1.0 and 5.0 A/g) [26]. The sodium-ion capacitors also exhibited high power performance, proving the potential of hybrid devices to make the most of both worlds: energy and power. Another MXene, V2CTx, can serve as a positive electrode in sodium-ion capacitors, versus hard carbon negative electrodes. The as-fabricated full cell exhibited high capacity, excellent cycling stability, and achieved an improvement in the voltage window up to 2.5 V [27]. The increase in the voltage window directly relates to improved energy density. Scientists in the University of Waterloo, Canada, used MXenes as sulfur hosts for lithium-sulfur batteries owing to their high underlying metallic conductivity and self-functionalized surfaces. The 70 wt.% S/Ti2CTx cathodes showed excellent cycling performance with specific capacity close to 1200 mAh/g at a five hour charge/discharge (C/5) and a capacity retention of 80 percent was achieved after 400 cycles at lower currents [28].
Investigations on using MXenes for other battery applications, such as magnesium-, aluminum- and sodium-ion, are also being carried out to understand how MXenes behave in alternative ion systems.
Future Outlook
The DoD seeks reliable, sustainable, and efficient materials to enable next-generation applications and improve the existing technologies. It is clear these materials possess properties beneficial to energy storage applications; however, MXenes are foreseen to be useful in many functional applications (see Figure 4). Very recently, MXenes were used to fabricate an all solid-state micro-supercapacitor [29], showing the promise of the material toward powering on-chip electronic devices. Mo2CTx MXene [4] as well as Mo2TiC2Tx MXene [30] showed semiconductor-like behavior, while Ti3C2Tx exhibited metallic behavior, suggesting the bandgap of these materials depends on the transition metal present. It was also shown by density-functional theory that the band gap can be tuned by changing the surface termination of MXenes [31].
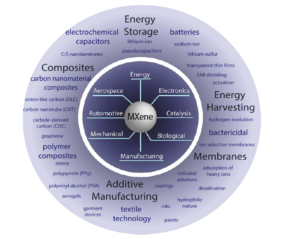
Figure 4. Schematic representing MXene properties and their use in current and future applications. (Released)
Furthermore, delaminated MXene is stable in a colloidal solution [12] enabling production of inks for additive manufacturing, conductive coatings and paints. By spray coating [15], spin casting [10] or other deposition methods, transparent thin films with optoelectronic properties could be produced, enabling high-quality displays or photovoltaics. As proven by mixing MXene with other materials, such as polymers or carbon nanoparticles, hybridization may be the key to unlocking functional materials which can provide mechanical strength while also providing energy storage or conversion, such as actuation or electromagnetic shielding. Initial biological studies have been conducted yielding Ti3C2Tx membranes, which exhibit ion-selective behavior [32] as well as bactericidal properties [33]. Smart textiles or armor technology could be enhanced by the incorporation of MXene materials through the coating of fibers or manufacturing MXene-loaded fibers. Additionally, vapor phase synthesis of MXenes would open new avenues for electronic applications of MXenes [34].
In addition to ‘A’ elements, the ‘M’ elements can also be selectively removed from the MAX phases (see Table 1), leading to the formation of ‘AX’ nanolaminates (see Figure 1). For example, applying a positive potential (electrochemical etching) on the Ti2SC MAX phase in aqueous electrolyte leads to the selective removal of Ti, leaving C/S nanolaminates [8]. The uniform distribution of sulfur in carbon frameworks, strong bonding between carbon and sulfur, and the layered structure of C/S nanolaminates afford their great potentials as cathode materials for lithium-sulfur batteries. Li-S batteries can store about four times more energy compared to Li-ion batteries, but the main challenge with lithium-sulfur technology is the reliability and lifetime of the cathode [35]. When testing the as-produced carbon/sulfur nanolaminates as cathode materials for lithium-sulfur batteries, the C/S nanolaminates achieved a high capacity of approximately 900 mAh/g and much better cycling stability compared to graphene/S nanostructures, a similar layered material. Further investigations showed that the electrochemical selective extraction of Ti can also be achieved from a number of other MAX phases, such as Ti3AlC2, Ti3SnC2 and Ti2GeC, creating a new class of ‘AX’ materials. The various ‘A’ and ‘X’ combinations known render the ‘AX’ structures highly attractive for a number of potential applications, such as electrical energy storage and catalysis.
With many configurations yet to be fully investigated, and MXene lending itself to be a material-by-design, numerous opportunities to improve current and create new technologies are available. Through continued optimization and discovery, the 2D metal carbides, nitrides and carbonitrides may find a place as versatile and multifunctional materials in a widespread array of defense applications. ■
References
1. Bonaccorso, F., Colombo, L., Yu, G., Stoller, M., Tozzini, V., Ferrari, A. C., Ruoff, R. S., & Pellegrini, V. (2015). Graphene, related two-dimensional crystals, and hybrid systems for energy conversion and storage. Science, 347(6217), 1246501. doi:10.1126/science.1246501.
2. Gogotsi, Y. (2014). What Nano Can Do for Energy Storage. ACS Nano, 8(6), 5369- 5371. doi:10.1021/nn503164x.
3. Naguib, M., Kurtoglu, M., Presser, V., Lu, J., Niu, J., Heon, M., Hultman, L., Gogotsi, Y., & Barsoum, M. W. (2011). Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Advanced Materials, 23(37), 4248-4253. doi:10.1002/adma.201102306.
4. Halim, J., Kota, S., Lukatskaya, M. R., Naguib, M., Zhao, M.-Q., Moon, E. J., Pitock, J., Nanda, J., May, S. J., Gogotsi, Y., & Barsoum, M. W. (2016). Synthesis and Characterization of 2D Molybdenum Carbide (MXene). Advanced Functional Materials, 26(18), 3118-3127. doi:10.1002/adfm.201505328.
5. Barsoum, M. W. (2013). MAX Phases: Properties of Machinable Ternary Carbides and Nitrides. Hoboken, NJ: Wiley.
6. Naguib, M., Mochalin, V. N., Barsoum, M. W., & Gogotsi, Y. (2014). 25th anniversary article: MXenes: a new family of two-dimensional materials. Advanced Materials, 26(7), 992-1005. doi:10.1002/adma.201304138.
7. Naguib, M., & Gogotsi, Y. (2015). Synthesis of two-dimensional materials by selective extraction. Accounts of Chemical Research, 48(1), 128-135. doi:10.1021/ar500346b.
8. Zhao, M. Q., Sedran, M., Ling, Z., Lukatskaya, M. R., Mashtalir, O., Ghidiu, M., Dyatkin, B., Tallman, D. J., Djenizian, T., Barsoum, M. W., & Gogotsi, Y. (2015). Synthesis of Carbon/Sulfur Nanolaminates by Electrochemical Extraction of Titanium from Ti2SC. Angewandte Chemie-International Edition, 54(16), 4810-4814. doi:10.1002/anie.201500110.
9. Eklund, P., Beckers, M., Jansson, U., Högberg, H., & Hultman, L. (2010). The Mn+1AXn phases: Materials science and thin-film processing. Thin Solid Films, 518(8), 1851-1878. doi:10.1016/j.tsf.2009.07.184.
10. Dillon, A. D., Ghidiu, M. J., Krick, A. L., Griggs, J., May, S. J., Gogotsi, Y., Barsoum, M. W., & Fafarman, A. T. (2016). Highly Conductive Optical Quality Solution-Processed Films of 2D Titanium Carbide. Advanced Functional Materials. doi:10.1002/adfm.201600357.
11. Lukatskaya, M. R., Mashtalir, O., Ren, C. E., Dall’Agnese, Y., Rozier, P., Taberna, P. L., Naguib, M., Simon, P., Barsoum, M. W., & Gogotsi, Y. (2013). Cation intercalation and high volumetric capacitance of two-dimensional titanium carbide. Science, 341(6153), 1502-1505. doi:10.1126/science.1241488.
12. Mashtalir, O., Naguib, M., Mochalin, V. N., Dall’Agnese, Y., Heon, M., Barsoum, M. W., & Gogotsi, Y. (2013). Intercalation and delamination of layered carbides and carbonitrides. Nature Communications, 4, 1716. doi:10.1038/ncomms2664.
13. Ghidiu, M., Lukatskaya, M. R., Zhao, M. Q., Gogotsi, Y., & Barsoum, M. W. (2014). Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature, 516(7529), 78-81. doi:10.1038/nature13970.
14. Mashtalir, O., Lukatskaya, M. R., Zhao, M. Q., Barsoum, M. W., & Gogotsi, Y. (2015). Amine-Assisted Delamination of Nb2C MXene for Li-Ion Energy Storage Devices. Advanced Materials, 27(23), 3501-3506. doi:10.1002/adma.201500604.
15. Hantanasirisakul, K., Zhao, M.-Q., Urbankowski, P., Halim, J., Anasori, B., Kota, S., Ren, C. E., Barsoum, M. W., & Gogotsi, Y. (2016). Fabrication of Ti3C2Tx MXene Transparent Thin Films with Tunable Optoelectronic Properties. Advanced Electronic Materials, 2, 1600050. doi:10.1002/aelm.201600050.
16. Jost, K., Perez, C. R., McDonough, J. K., Presser, V., Heon, M., Dion, G., & Gogotsi, Y. (2011). Carbon coated textiles for flexible energy storage. Energy & Environmental Science, 4(12), 5060. doi:10.1039/c1ee02421c.
17. Jost, K., Stenger, D., Perez, C. R., Mc-Donough, J. K., Lian, K., Gogotsi, Y., & Dion, G. (2013). Knitted and screen printed carbon-fiber supercapacitors for applications in wearable electronics. Energy & Environmental Science, 6(9), 2698-2705. doi:10.1039/c3ee40515j.
18. Ling, Z., Ren, C. E., Zhao, M. Q., Yang, J., Giammarco, J. M., Qiu, J. S., Barsoum, M.W., & Gogotsi, Y. (2014). Flexible and conductive MXene films and nanocomposites with high capacitance. Proceedings of the National Academy of Sciences of the United States of America, 111(47), 16676-16681.doi:10.1073/pnas.1414215111.
19. Zhao, M. Q., Ren, C. E., Ling, Z., Lukatskaya, M. R., Zhang, C. F., Van Aken, K. L., Barsoum, M. W., & Gogotsi, Y. (2015). Flexible MXene/Carbon Nanotube Composite Paper with High Volumetric Capacitance. Advanced Materials, 27(2), 339-345.doi:10.1002/adma.201404140.
20. Simon, P., Gogotsi, Y., & Dunn, B. (2014). Where Do Batteries End and Supercapacitors Begin? Science, 343(6176), 1210-1211. doi:10.1126/science.1249625.
21. Department of Defense Fiscal Year (FY) 2015 Budget Estimates. (2014). Defense Wide Justification Book.
22. Naguib, M., Come, J., Dyatkin, B., Presser, V., Taberna, P. L., Simon, P., Barsoum, M. W., & Gogotsi, Y. (2012). MXene: a promising transition metal carbide anode for lithium-ion batteries. Electrochemistry Communications, 16(1), 61-64. doi:10.1016/j.elecom.2012.01.002.
23. Luo, J., Tao, X., Zhang, J., Xia, Y., Huang, H., Zhang, L., Gan, Y., Liang, C., & Zhang, W. (2016). Sn(4+) Ion Decorated Highly Conductive Ti3C2 MXene: Promising Lithium-Ion Anodes with Enhanced Volumetric Capacity and Cyclic Performance. ACS Nano, 10(2), 2491-2499. doi:10.1021/acsnano.5b07333.
24. Ren, C. E., Zhao, M. Q., Makaryan, T., Halim, J., Boota, M., Kota, S., Anasori, B., Barsoum, M. W., & Gogotsi, Y. (2016). Porous Two-Dimensional Transition Metal Carbide (MXene) Flakes for High-Performance Li-Ion Storage ChemElectroChem, 3(5), 689–693. doi:10.1002/celc.201600059.
25. Boota, M., Anasori, B., Voigt, C., Zhao, M. Q., Barsoum, M. W., & Gogotsi, Y. (2016). Pseudocapacitive Electrodes Produced by Oxidant-Free Polymerization of Pyrrole between the Layers of 2D Titanium Carbide (MXene). Advanced Materials, 28(7), 1517-1522. doi:10.1002/adma.201504705.
26. Wang, X., Kajiyama, S., Iinuma, H., Hosono, E., Oro, S., Moriguchi, I., Okubo, M., & Yamada, A. (2015). Pseudocapacitance of MXene nanosheets for high-power sodium-ion hybrid capacitors. Nature Communications, 6, 6544. doi:10.1038/ncomms7544.
27. Dall’Agnese, Y., Taberna, P. L., Gogotsi, Y., & Simon, P. (2015). Two-Dimensional Vanadium Carbide (MXene) as Positive Electrode for Sodium-Ion Capacitors. Journal of Physical Chemistry Letters, 6(12), 2305-2309. doi:10.1021/acs.jpclett.5b00868.
28. Liang, X., Garsuch, A., & Nazar, L. F. (2015). Sulfur cathodes based on conductive MXene nanosheets for high-performance lithium-sulfur batteries. Angewandte Chemie International Edition, 54(13), 3907-3911. doi:10.1002/anie.201410174.
29. Shen, B.-S., Wang, H., Wu, L.-J., Guo, R.-S., Huang, Q., & Yanx, X.-B. (2016). All-solid-state flexible microsupercapacitor based on two-dimensional titanium carbide. Chinese Chemical Letters. doi:10.1016/j.cclet.2016.04.012.
30. Anasori, B., Shi, C., Moon, E. J., Xie, Y., Voigt, C. A., Kent, P. R. C., May, S. J., Billinge, S. J. L., Barsoum, M. W., & Gogotsi, Y. (2016). Control of electronic properties of 2D carbides (MXenes) by manipulating their transition metal layers. Nanoscale Horizons, 1(3), 227-234. doi:10.1039/c5nh00125k.
31. Tang, Q., Zhou, Z., & Shen, P. (2012). Are MXenes promising anode materials for Li ion batteries? Computational studies on electronic properties and Li storage capability of Ti3C2 and Ti3C2X2 (X = F, OH) monolayer. Journal of the American Chemical Society, 134(40), 16909-16916. doi:10.1021/ja308463r.
32. Ren, C. E., Hatzell, K. B., Alhabeb, M., Ling, Z., Mahmoud, K. A., & Gogotsi, Y. (2015). Charge- and Size-Selective Ion Sieving Through Ti3C2Tx MXene Membranes. Journal of Physical Chemistry Letters, 6(20), 4026-4031. doi:10.1021/acs.jpclett.5b01895.
33. Rasool, K., Helal, M., Ali, A., Ren, C. E., Gogotsi, Y., & Mahmoud, K. A. (2016). Antibacterial Activity of Ti3C2Tx MXene. ACS Nano, 10(3), 3674-3684. doi:10.1021/acsnano.6b00181.
34. Gogotsi, Y. (2015). Chemical vapour deposition: Transition metal carbides go 2D. Nature Materials, 14(11), 1079-1080. doi:10.1038/nmat4386.
35. Bruce, P. G., Freunberger, S. A., Hardwick, L. J., & Tarascon, J. M. (2012). Li-O2 and Li-S batteries with high energy storage. Nature Materials, 11(1), 19-29. doi:10.1038/nmat3191.
Kathleen Maleski is a Ph.D. student at Drexel University where she researches for the A.J. Drexel Nanomaterials Institute. Primarily, she studies colloidal solutions of carbon and 2D nanomaterials, in addition to their applications in energy storage. Her background also includes computational research on graphene with the Materials Research Science and Engineering Center at Penn State University. In 2014, she obtained a Bachelor of Science degree in physics and a minor in chemistry from Washington College, Md.
Meng-Qiang Zhao, Ph.D., is a post-doctoral researcher at Drexel University and the A.J. Drexel Nanomaterials Institute. He works on synthesis and manufacturing of two-dimensional transition metal carbides (MXenes)-based composite materials and their energy-related applications. His research experience includes synthesis of carbon nanomaterials, especially carbon nanotubes as well as hierarchical nanocomposites, energy storage systems such as Li-S batteries, and supercapacitors. He obtained his Ph.D. in 2013 and his Bachelor of Science in 2008 from Tsinghua University, Beijing, China, in chemical engineering.
Professor Yury Gogotsi is a Distinguished University Professor and Trustee Chair of Materials Science and Engineering at Drexel University. He is also the founding director of the A.J. Drexel Nanomaterials Institute and associate editor of ACS Nano. He has a Ph.D. in physical chemistry from Kiev Polytechnic, D.Sc. in materials engineering from the Ukrainian Academy of Sciences and Dr.h.c. degree from Paul Sabatier University, Toulouse, France. He works on nanostructured carbons and two-dimensional carbides for energy related and biomedical applications. He has received numerous national and international awards for his research, was recognized as Highly Cited Researcher by Thomson-Reuters in 2014 and 2015, and elected a Fellow of AAAS, MRS, RSC, ECS and ACerS and a member of the World Academy of Ceramics.


