Arthropod-borne viruses, known as arboviruses, pose an immense healthcare burden in developing and undeveloped countries in the tropical and sub-tropical regions of the world [1]. These diseases are mostly transmitted via mosquito bites, but ticks, gnats, and fleas can also spread some of the diseases [1]. Over the past several years, worldwide outbreaks of Dengue virus (DENV), Yellow fever virus (YFV), Chikungunya virus (CHIKV), Japanese encephalitis virus (JEV) and Zika virus (ZIKV) have not only claimed millions of lives, but also strained global healthcare infrastructures. Moreover, the lack of available (ZIKV, CHIKV) or effective (DENV, YFV and JEV) vaccines, along with the absence of specific treatment, makes these infections a global problem. These infections remain a concern to the U.S. Department of Defense (DoD), since U.S. warfighters are deployed in many of the regions where these viruses are present (see Figure 1).
Protecting Deployed Warfighters
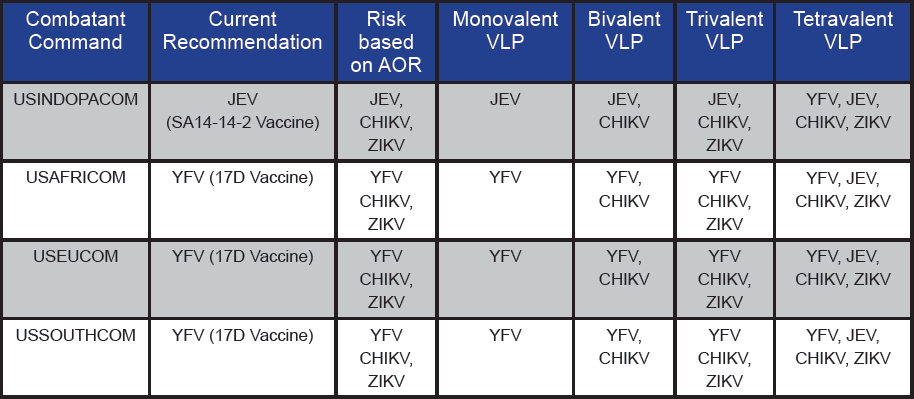
Defending the warfighter against all types of biological threats is a key mission of the DoD, which is why regulations have been developed regarding immunizations and chemoprophylaxis for infection prevention. These regulations recommend the vaccination of warfighters against arboviruses based on Area or Responsibility (AOR) (see Table 1). These recommendations include vaccination against YFV and JEV, as vaccines for these diseases are available.
However, ZIKV and CHIKV are also prevalent in regions of U.S. troop deployment, justifying the development of a vaccine for these diseases. Specifically, CHIKV infections can be highly debilitating due to associated arthral gia, resulting in joint pain that can last from months to years in duration [2]. Similarly, ZIKV has been associated with Guillain Barre Syndrome, a disorder that can result in weakness of the arms and legs that can last from weeks to months [3]. Although vaccines for JEV and YFV are available, these vaccines are largely derived from live attenuated viruses (LAV) and have their own limitations [4]. LAV vaccines are contraindicated in certain groups of individuals due to safety concerns, and in some cases, adverse reactions [5, 6]. Also, due to their monovalent nature, each vaccine has to be administered separately, thereby increasing the number of required immunizations. A multivalent vaccine providing protection against CHIKV, ZIKV, YFV, and JEV is ideally suited for use by the DoD due to reduction in number of immunizations and protection against four major arboviruses (see Table 1). The deployment of U.S. warfighters to various regions of the world can be unpredictable and often expedited. Hence, protecting warfighters against these diseases in advance and ensuring readiness for deployment anywhere in the world would be a major advantage.
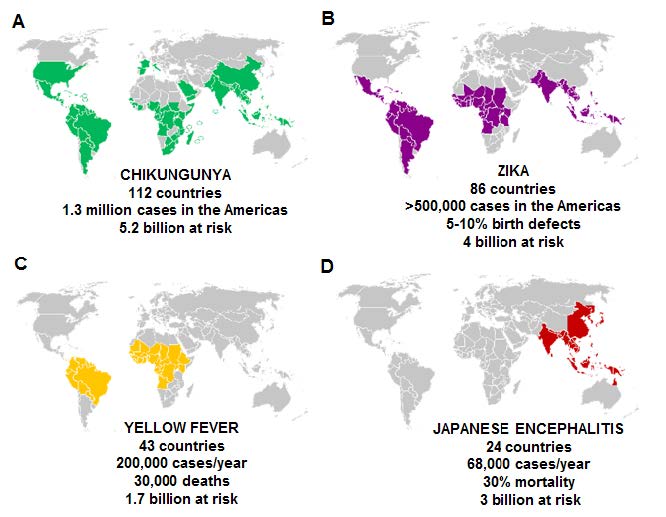
Figure 1. Global incidence, estimated risk and mortality of arboviral diseases targeted by the multivalent vaccine
Table 1. Current recommendations for vaccination of U.S. warfighters based on AOR and the possible benefit of multivalent arboviral VLP vaccines based on risk
Arboviruses and the Need for a Multivalent Vaccine
While research interest in arboviral diseases has grown in recent years, most of the work has focused on Dengue virus. This is in part because Dengue is the most prevalent arbovirus infecting humans, causing an estimated 100 million infections annually [7, 8]. Other arboviruses, such as CHIKV, JEV, YFV and ZIKV, have attracted limited investment from the medical research community—suggesting that they be considered neglected tropical diseases [9]. Nevertheless, these viruses remain a major concern and are responsible for infecting millions of people worldwide (see Figure 1).
CHIKV circulates in 112 countries, with a combined population of 5.2 billion people at risk, and has caused an estimated 1.3 million cases in just the Americas. While CHIKV may not be associated with a high mortality rate, it causes a debilitating infection that affects joints and movement [10] that can last for years. On the other hand, infections like JEV not only put approximately 3 billion people at risk, but mortality rates can reach 30 percent—with the most severe outcome in children (see Figure 1).
The recent outbreaks of ZIKV infection posed a severe health concern due to its devastating effects on an the unborn fetus [11], the possible sexual transmission of the virus, and long term virus shedding [12]. ZIKV currently circulates in 81 countries, with 500,000+ cases reported in the Americas during the 2015–2017 outbreak. Yellow fever remains a major concern in 43 countries in South America and Africa, with more than 1.7 billion at risk. Strict vaccination guidelines for travelers to and from the YFV-prone countries has managed to limit the spread of the virus. However, as global trade and travel grows in volume, these restrictions may not be sufficient to curtail the spread of the virus in the future.
Significant progress has been made on the Dengue vaccine front with the approval by the European Commission of a tetravalent live attenuated vaccine (Dengvaxia) developed by Sanofi Pasteur [13]. This vaccine has undergone extensive clinical testing which has highlighted some of its limitations—including suboptimal response in people previously uninfected with DENV [14]. Hence, the current recommendation is to vaccinate individuals that have previously been exposed to DENV.
Similar DENV tetravalent LAV vaccines are currently under development [15]. However, some of the limitations of the LAV platform, highlighted by Dengvaxia studies, may plague other such vaccine candidates. This fact raises concerns regarding the potential success of other live attenuated multivalent arboviral vaccines, and warrants the development of alternate vaccine platforms.
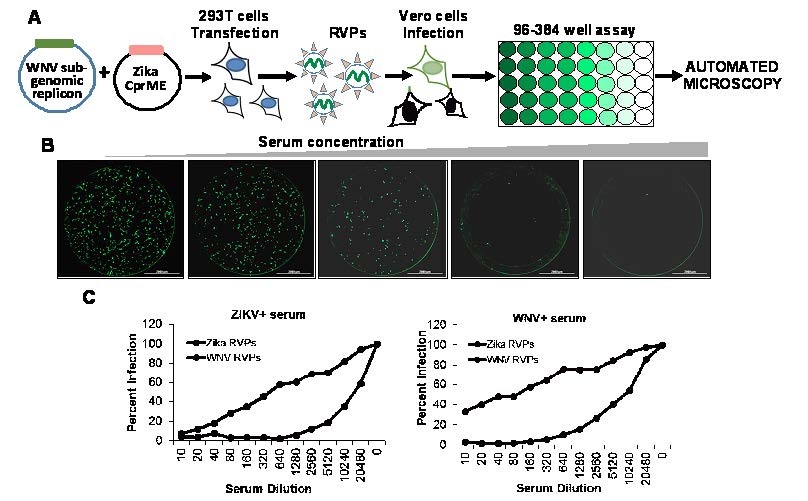
Figure 2. RVP based neutralization assay. (A) Strategy for production of RVPs using two plasmid system and testing neutralization using automated microscopy. (B) Single round infection of Vero cells by RVPs in the presence of varying concentrations of ZIKV specific serum. (C) RVP based neutralization assay can differentiate between different closely related viruses like ZIKV and WNV. RVP assay was used to test serum from either a WNV or ZIKV infected individual.
Increasing Risk Factors for the Spread of Arboviral Diseases
Major outbreaks of arboviral diseases across continents has become a yearly phenomenon [16]. The distribution and rapid spread of arboviral diseases is regulated by multiple factors, including changes in global temperature, higher volumes of global trade and travel, and increased urbanization accompanied by deforestation [17]. The global distribution of the Aedes genus, the primary vector for many arboviruses, is on the rise partly due to regional temperature changes [18]. The introduction of Asian lineages of CHIK and ZIKV to the Americas exemplifies such a spread [19, 20]. Other factors, like urbanization of tropical regions and deforestation, result in closer contact between humans and zoonotic reservoirs of arboviruses, such as non-human primates [17]. Moreover, increased temperature and flooding affects the vector population and makes vector control challenging [21]. Lack of proper waste disposal and sanitation in under-developed countries further exacerbates these problems. These factors call for routine vaccination against arboviral diseases in endemic areas.
Approach: Virus-like Particle Vaccines
Researchers at the Texas Tech University Health Sciences Center (TTUHSC) are developing virus-like particle (VLP)-based vaccines for these arboviral diseases. VLPs are non-infectious virus particles produced in vitro in different host expression systems (like mammalian cells) by expressing the structural proteins of viruses [22]. These proteins self-assemble into VLPs that lack a genome, but closely resemble native virus. They are both safe and highly immunogenic. As the VLPs are non-infectious, due to a lack of genome, they do not need to be inactivated with formalin—unlike Purified Inactivated Vaccines derived from live virus.
Furthermore, the antigenicity of these particles can be beneficial, as the conformational changes associated with formalin inactivation are avoided. The TTUHSC team is developing stable cell lines that secrete VLPs for ZIKV, CHIKV, YFV and JEV. These cell lines continuously secrete copious amounts of VLPs that can be harvested from culture supernatants and scaled up for mass production. A similar strategy has been used for the successful multivalent VLP vaccine against Human Papilloma Virus, suggesting that this strategy is commercially viable [23].
Concurrent with the development of a vaccine is the need to develop a sensitive, high throughput, and reproducible assay that can be used to test vaccine efficacy by measuring neutralizing antibody titers. In this regard, the Plaque Reduction Neutralization Test (PRNT) has been the gold standard for testing vaccine efficacy [24]. However, classical PRNT assays require handling infectious virus in a Biosafety Level 3 (BSL-3) containment environment, are tedious, and usually require up to a week of incubation. In fact, one of the major obstacles of undertaking arbovirus research is the requirement of BSL-3 containment for infectious virus studies.
These restrictions can be overcome by the use of Reporter Virus Particle (RVP)-based assay that eliminates the need for infectious virus [25]. The RVP assays work by dividing the genome of the virus in two separate plasmids. One provides the non-structural proteins and a reporter gene like green fluorescent protein (GFP), while the other plasmid provides the structural proteins (see Figure 2A).
In vitro complementation of the two plasmids results in generation of RVPs that carry a partial genome and are capable of a single round of infection. Infection of cells with the RVPs results in labeling of cells with GFP that can be easily quantified using automated microscopy (see Figure 2B). The assay is highly specific and can differentiate between closely related viruses like ZIKV and West Nile virus (WNV) using specific serum (see Figure 2C). Similar RVP assays are also available for CHIKV [26].
The RVP assay can be used to study viral lifecycle, including viral entry, testing of neutralizing antibodies, and screening for antiviral compounds. Arbovirus research is further complicated by cross-reactivity between viruses, which makes differential diagnosis a challenge in sero-surveillance studies [27]. In this regard, the RVP assay can provide much needed specificity to differentiate between related arboviruses for biosurveillance studies.
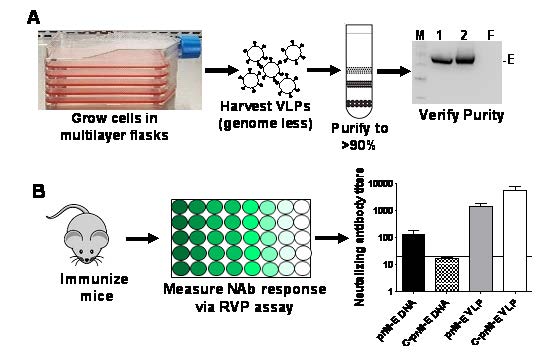
Figure 3. Strategy for development of a VLP vaccine for arboviruses followed by efficacy studies in mice. (A) Stable cells lines constitutively secreting genome less VLPs were grown in multi-layer flasks. The VLPs were concentrated by ultracentrifugation, further purified and analyzed via western blotting for the ZIKV E protein. M=marker; 1, 2=concentrated VLPs; F= filtrate. (B) Mice were immunized with the above purified VLPs and sera harvested. Production of neutralizing antibodies was determined using the RVP assay. Graph depicts neutralizing antibody titers produced in mice immunized with prM-E or C-prM-E DNA versus prM-E or C-prM-E VLPs.
Current Research
In preliminary studies, researchers at TTUHSC developed a VLP vaccine and an RVP assay against ZIKV [28]. In this study, the researchers expressed ZIKV structural proteins in mammalian cells that resulted in production of VLPs. These VLPs were harvested from culture supernatants, purified, and used to immunize mice. The results showed that the VLP vaccine induced potent neutralizing antibodies that were of higher titer than other platforms, such as a DNA vaccine (see Figure 3). The inclusion of the capsid, C protein in the VLPs also enhanced neutralizing antibody titers. The use of stable cell lines to produce VLPs provides a much needed advancement of this platform making it scalable and economical for commercial development.
The researchers also optimized an RVP assay for detecting ZIKV neutralizing antibodies. This study showed that the results of the RVP assay mirror closely with assays using infectious virus, suggesting that the RVP-based diagnostic assay can replace classical PRNT assays [28]. The research team at TTUHSC has also developed similar RVP assays for other arboviruses, including CHIKV, JEV and YFV.
Recommendations for Further Research
Building on the success of the ZIKV VLP vaccine study, researchers at TTUHSC have developed cell lines that secrete VLPs for JEV, YFV, or CHIKV. The cell lines were generated via transduction of 293T cells with commonly used lentiviral vector expressing arboviral structural proteins. This popular biotechnology tool allows for the stable integration of a desired gene into the cells’ genome and the incorporation of a selection marker like Blasticidin. The cells were then sub-cloned to generate single-cell clones that continually secrete high levels of VLPs and are stable even when cultured in the absence of Blasticidin. VLPs harvested from culture supernatants can be purified and used in various vaccine formulations.
Developing a multivalent vaccine by combining individual VLP components has three advantages:
- Multivalent vaccines can be tailored to different geographical regions based on the prevalence of certain infections in the area (see Table 1).
- The antigenic dose of individual VLP components can be adjusted depending on the kind and level of immune response elicited against each antigen. The antigenic dose is difficult to control in LAV vaccines due to difficulty in predicting how the attenuated virus will replicate in different individuals, as evident in the tetravalent LAV dengue vaccine studies.
- Booster doses can be tailored depending on the kind of immune response generated after the primary immunization. The use of the RVP assay to quickly determine neutralizing response against each virus will provide much needed information on how to balance the immune response against each.
Developing a Multivalent Vaccine
The development of a multivalent VLP vaccine will require the careful testing of each vaccine component. Monovalent VLPs in varying doses will be tested in mice to determine the optimal dose for a protective immune response against each virus. Subsequent testing of the VLPs in different combinations—such as bivalent, trivalent, and tetravalent vaccines appropriate for different geographical regions—will need to be conducted (see Table 1). The combination of VLPs may possibly skew the response towards a specific antigen. Hence, adjusting the dose of each VLP in a multivalent preparation will be needed to obtain a balanced broad-spectrum response.
Future studies should determine whether a single administration of vaccine is sufficient, or a booster immunization would be required to maximize the strength and duration of immune response. These studies will pave the way for inoculation experiments in appropriate small animal models using infectious virus to validate vaccine efficacy. Regulatory approval of a new drug/vaccine candidates in the U.S. requires testing in at least two different animal species. Future studies will be aimed at conducting vaccine efficacy studies in non-human primates as they closely recapitulate the characteristics of human infection, making them ideal candidates for immunological and inoculation experiments.
Preparing the Vaccine for Human Use
One of the primary factors driving vaccine commercialization is the cost of production, especially when the vaccines are intended for underdeveloped parts of the world. In this regard, the development of stable cell lines that continuously secrete high concentrations of VLPs provides a much needed advantage. The researchers at TTUHSC have developed a protocol for the bulk production of VLPs in multi-layer flasks, followed by VLP concentration and purification using Optiprep (Iodixanol) gradient to yield high purity VLPs (see Figure 2).
Further scale-up of vaccine production—especially for human use—can be achieved by expanding cells in bioreactors and production under good manufacturing practices. Protocols will also be established for VLP concentration and purification for large-scale production, like Tangential Flow Filtration (TFF) followed by ultracentrifugation for high purity VLPs. These scale-up and purification protocols for virus particles are routinely used by vaccine manufacturers and can be easily adapted for our multivalent vaccine production.
Conclusion
The worldwide risk of arboviral diseases is on the rise [29]. Arboviral diseases pose a significant threat to the human population, yet most remain neglected primarily due to a lack of funding. CHIKV, ZIKV, YFV, and JEV are all arboviruses that are prevalent in AORs where DoD personnel operate. A multivalent vaccine is ideally suited for protecting the warfighter from these rising biological threats. The VLP platform provides many advantages over conventional vaccines, including enhanced safety, efficacy, and economical scalability. Along with the VLP vaccine, an RVP assay would provide much needed advancement in detecting vaccine efficacy and it would provide an easy and rapid platform for bio-surveillance studies in regions where U.S. forces are stationed.
References
- Weaver, S. C., & Reisen, W. K. (2010). Present and future arboviral threats. Antiviral Research, 85(2), 328–345. doi:10.1016/j. antiviral.2009.10.008
- Aalst, M. V., Nelen, C. M., Goorhuis, A., Stijnis, C., & Grobusch, M. P. (2017). Longterm sequelae of chikungunya virus disease: A systematic review. Travel Medicine and Infectious Disease, 15, 8–22. doi:10.1016/j. tmaid.2017.01.004
- Dirlikov, E., Major, C. G., Medina, N. A., Lugo-Robles, R., Matos, D., Muñoz-Jordan, J. L., . . . Rivera-García, B. (2018). Clinical features of Guillain-Barré Syndrome with vs without Zika virus infection, Puerto Rico, 2016. JAMA Neurology, 75(9), 1089. doi:10.1001/jamaneurol.2018.1058
- Barrett, A. D. (2017). Yellow fever live attenuated vaccine: A very successful live attenuated vaccine but still we have problems controlling the disease. Vaccine, 35(44), 5951–5955. doi:10.1016/j.vaccine.2017.03.032
- Alexander, K. E., Tong, P. L., Macartney, K., Beresford, R., Sheppeard, V., & Gupta, M. (2018). Live zoster vaccination in an immunocompromised patient leading to death secondary to disseminated varicella zoster virus infection. Vaccine, 36(27), 3890–3893. doi:10.1016/j.vaccine.2018.05.078
- Wiley, C. C. (2015). Immunizations: Vaccinations in general. Pediatrics in Review, 36(6), 249–259. doi:10.1542/pir.36-6-249
- Bhatt, S., Gething, P. W., Brady, O. J., Messina, J. P., Farlow, A. W., Moyes, C. L., . . . Hay, S. I. (2013). The global distribution and burden of dengue. Nature, 496(7446), 504–507. doi:10.1038/nature12060
- Brady, O. J., Gething, P. W., Bhatt, S., Messina, J. P., Brownstein, J. S., Hoen, A. G., . . . Hay, S. I. (2012). Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Neglected Tropical Diseases, 6(8). doi:10.1371/journal. pntd.0001760
- LaBeaud, A. D. (2008). Why arboviruses can be neglected tropical diseases. PLoS Neglected Tropical Diseases, 2(6). doi:10.1371/journal.pntd.0000247
- Yactayo, S., Staples, J. E., Millot, V., Cibrelus, L., & Ramon-Pardo, P. (2016). Epidemiology of Chikungunya in the Americas. Journal of Infectious Diseases, 214(Suppl 5). doi:10.1093/infdis/jiw390
- Pierson, T. C., & Diamond, M. S. (2018). The emergence of Zika virus and its new clinical syndromes. Nature, 560(7720), 573–581. doi:10.1038/s41586-018-0446-y
- Mead, P. S., Duggal, N. K., Hook, S. A., Delorey, M., Fischer, M., Mcguire, D. O., . . . Hinckley, A. F. (2018). Zika virus shedding in semen of symptomatic infected men. New England Journal of Medicine, 378(15), 1377–1385. doi:10.1056/nejmoa1711038
- Halstead, S. B., & Russell, P. K. (2016). Protective and immunological behavior of chimeric yellow fever dengue vaccine. Vaccine, 34(14), 1643–1647. doi:10.1016/j.vaccine.2016.02.004
- Dans, A. L., Dans, L. F., Lansang, M. A., Silvestre, M. A., & Guyatt, G. H. (2018). Controversy and debate on dengue vaccine series—paper 3: Final response to review of a licensed dengue vaccine: Inappropriate subgroup analyses and selective reporting may cause harm in mass vaccination programs. Journal of Clinical Epidemiology, 95, 142. doi:10.1016/j.jclinepi.2017.12.025
- Tripathi, N. K., & Shrivastava, A. (2018). Recent developments in recombinant protein–based Dengue vaccines. Frontiers in Immunology, 9. doi:10.3389/fimmu.2018.01919
- Gubler, D. J. (2002). The global emergence/ resurgence of arboviral iseases as public health problems. Archives of Medical Research, 33(4), 330–342. doi:10.1016/s0188- 4409(02)00378-8
- Liang, G., Gao, X., & Gould, E. A. (2015). Factors responsible for the emergence of arboviruses; strategies, challenges and limitations for their control. Emerging Microbes & Infections, 4(3). doi:10.1038/emi.2015.18
- Kraemer, M. U., Sinka, M. E., Duda, K. A., Mylne, A. Q., Shearer, F. M., Barker, C. M., . . . Hay, S. I. (2015). The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. ELife, 4. doi:10.7554/elife.08347
- Leparc-Goffart, I., Nougairede, A., Cassadou, S., Prat, C., & Lamballerie, X. D. (2014). Chikungunya in the Americas. The Lancet, 383(9916), 514. doi:10.1016/s0140- 6736(14)60185-9
- Musso, D. (2015). Zika virus transmission from French Polynesia to Brazil. Emerging Infectious Diseases, 21(10), 1887–1887. doi:10.3201/eid2110.151125
- Tabachnick, W. J. (2016). Climate change and the arboviruses: Lessons from the evolution of the Dengue and Yellow Fever viruses. Annual Review of Virology, 3(1), 125-145. doi:10.1146/annurev-virology-110615-035630
- Mohsen, M. O., Zha, L., Cabral-Miranda, G., & Bachmann, M. F. (2017). Major findings and recent advances in virus–like particle (VLP)-based vaccines. Seminars in Immunology, 34, 123–132. doi:10.1016/j. smim.2017.08.014
- Luxembourg, A., & Moeller, E. (2017). 9-Valent human papillomavirus vaccine: A review of the clinical development program. Expert Review of Vaccines, 16(11), 1119–1139. doi :10.1080/14760584.2017.1383158
- Kuno, G. (2003). Serodiagnosis of flaviviral infections and vaccinations in humans. Advances in Virus Research, 61, 3–65.
- Pierson, T. C., Sánchez, M. D., Puffer, B. A., Ahmed, A. A., Geiss, B. J., Valentine, L. E., . . . Doms, R. W. (2006). A rapid and quantitative assay for measuring antibody-mediated neutralization of West Nile virus infection. Virology, 346(1), 53–65. doi:10.1016/j.virol.2005.10.030
- Akahata, W., Yang, Z., Andersen, H., Sun, S., Holdaway, H. A., Kong, W., . . . Nabel, G. J. (2010). A virus-like particle vaccine for epidemic Chikungunya virus protects nonhuman primates against infection. Nature Medicine, 16(3), 334–338. doi:10.1038/ nm.2105
- Charrel, R. N. (2016). Diagnosis of arboviral infections – A quagmire of cross reactions and complexities. Travel Medicine and Infectious Disease, 14(1), 11–12. doi:10.1016/j. tmaid.2016.01.006
- Garg, H., Sedano, M., Plata, G., Punke, E. B., & Joshi, A. (2017). Development of virus-like-particle vaccine and reporter assay for Zika virus. Journal of Virology, 91(20). doi:10.1128/jvi.00834-17
- Young, P. R. (2018). Arboviruses: A family on the move. Advances in Experimental Medicine and Biology Dengue and Zika: Control and Antiviral Treatment Strategies, 1–10. doi:10.1007/978-981-10-8727-1_1


