Wireless physiological sensing in tactical environments has the potential to greatly enhance health outcomes in combat casualty care. Not only does it provide actionable data for field care; it can also supply personal health metrics and environmental data for use in downrange care, medical research, and strategic planning [1].
Such wireless sensing systems can also be used to detect warfighter fatigue, “thermal work strain [2],” chemical or biological exposure [3], and dynamic physical information that could be useful to a team operating in the field (e.g., activity classification, gesture monitoring) [4]. More broadly, wearable physiological status monitoring (PSM) devices represent a new class of military technologies that has the potential to improve readiness and “support decisions and actions in response to operating in a threat environment [5, 6].”
To meet this potential, such systems should (a) operate in real time, (b) function wirelessly, (c) be capable of multi-modal sensing, and (d) be compatible with existing situational awareness systems like Nett Warrior.
The development of wireless PSM technologies for tactical combat casualty care (TCCC) and warfighter readiness has involved the adaptation of commercial wearables [3, 1] as well as the pursuit of new research into novel material architectures and sensor technologies. The former includes commercial off-the shelf devices like smartwatches, activity/fitness trackers, and augmented reality headsets (including “smart glasses”). While these devices are readily available and offer plug-and-play functionality, they have limited sensing modalities and satisfy only a few of the requirements for tactical PSMs. Instead, progress in this space depends on achieving fundamental research and development (R&D) advancements in wearables technology. As described below, these efforts fall within the following categories: (a) novel biological and chemical sensors, (b) material architectures for stretchable, skin-mountable circuitry, and (c) methods for the integration of microelectronics for sensing, signal processing, and wireless data transmission.
Biological and Chemical Sensors
In recent years, researchers have achieved remarkable progress developing sensors that use lab-on-a-chip microfluidics, genetic engineering, and/or synthetic biology to detect biomarkers, toxins, and other biological or chemical analytes. These sensors typically measure analytes via electrochemical or optical/visual cues based on photonics or fluorescence [7]. The sweat-sensing patch discussed in Rose et al. [8] is illustrative of the state of advanced wearable biosensing technology.
The patch is a bandage-like electronic sticker that contains functionalized electrodes for measuring Na+ concentrations in sweat; a microfluicid paper pad for fluid and vapor transport; and radio-frequency identification (RFID) circuitry for the near field communication (NFC) of sensor readings to a nearby smartphone. Although this particular model only measures concentrations of sodium, its electrodes could be functionalized with an ion-selective polymer ionophore membrane to measure K+, Cl–, Mg2+, and other ions that can be used to establish baseline physiological states and monitor changes in the balance of electrolytes within sweat. Such multiplexed perspiration analysis was subsequently demonstrated in a multi-electrode patch capable of measuring glucose and lactate concentrations (see Figure 1A) [9]. In lieu of RFID-based communications, the patch interfaces with a smartphone via Bluetooth radio transceiver. While this allows for higher bandwidths in data transmission, it does require an on-board battery and electronics for power regulation. In both implementations, wearable electronic functionality is accomplished using a flexible printed circuit board (fPCB). Although not stretchable, fPCBs display adequate compliance for conformable contact with select parts of the body, including the wrist, forehead, or chest.
Another example of advanced biosensing is the hybrid sensor shown in Figure 1B, which combines electrophysiological and chemical biosensing modalities [10]. This sensor is composed of a thin, flexible polyester sheet connected to a Bluetooth transceiver, a printed circuit board for signal processing, and a series of screen-printed electrodes capable of both sweat lactate sensing and electrocardiography (ECG). Because of its mechanical compliance, the sensor can conform to the body, and is capable of simultaneously monitoring cardiac activity (e.g., heart rate) and lactate levels during perspiration. As with the other implementations discussed above, the sensors reported by Imani et al. [10] are flexible but not stretchable.
In general, mechanical flexibility is essential for ensuring close contact and acquiring accurate physiological status measurements. For some applications, however, flexibility is not enough—the sensor should also be stretchable and able to support high elastic strains. This allows the sensor to be placed on joints or rounded (i.e., non-developable) parts of the body without running the risk of impairing natural motion or delaminating. Stretchable functionality in a biosensor would also enable its incorporation into stretchable fabrics that can be pulled over the skin.
Therefore, R&D efforts in wearable PSM technology are increasingly focused on ways to combine biosensors with skinlike electronics that are soft and elastic, as well as thin and flexible.

Figure 1. (A) Multi-electrode sweat-sensing patch for more complete perspiration analysis [9]. (B) Flexible biosensor capable of ECG and sweat sensing [10].
Epidermal Electronics
One approach for achieving stretchable functionality is to create wearable circuits using wavy or serpentine wiring. This so-called deterministic approach to stretchable circuits is commonly used in epidermal electronics like the multi-modal wireless sensor shown in Figure 2A [11]. Such a circuit measures a variety of biosignals, including ECG, temperature, and strain, and transmits its data via NFC. As with the sweat sensing patch reported in Rose et al. [8], this circuit does not require an on-board battery, instead harvesting power through electromagnetic induction. Such multi-modal sensing allows for a wide range of PSM functionalities, such as tracking muscle contractions, cardiac monitoring, and electroencephalography for monitoring alpha wave oscillations associated with neural activity.
The serpentine shape of the traces allows the circuit to stretch or wrinkle while preserving the natural mechanics of the skin. Such patterning is accomplished using lithographic techniques that combine conventional clean-room microfabrication tools with unconventional materials and novel processing steps.
More recent efforts in epidermal electronics have focused on techniques that eliminate clean-room lithography in order to enable rapid, inexpensive fabrication. The human-computer interaction R&D community has adopted the popular approach of printing conductive inks on transferrable “temporary tattoo” film using standard commercial inkjet printers. Examples of this approach include the Skintillates and SkinMarks architectures reported in Lo et al. [12] and Weigel et al. [13], respectively. While these circuits are ultra-thin and allow for very close contact with the body, the circuit traces have limited conductivity and stretchability, and are less reliable than wiring for digital electronics.
Recently, our research group, the Soft Machines Lab at Carnegie Mellon University, partnered with the Soft and Printed Microelectronics Laboratory at the University of Coimbra (Portugal) to develop methods for the rapid and inexpensive fabrication of epidermal electronics that overcome some of the limitations of prior techniques [14]. In this materials architecture, the circuit is composed of thin films of liquid metal (LM) alloy coated on silver-based conductive ink traces printed on a temporary tattoo transfer film. The LM alloy is a non-toxic blend of gallium and indium, fully sealed by the tattoo film and thin coating of silicone elastomer. As shown in Figure 2B, the circuit adheres to skin and can be populated with microelectronic components.
The LM coating has two functional roles. First, it binds to the conductive ink and prevents the traces from cracking when stretched. Second, it acts as a room-temperature solder for connecting the pins of the surface-mounted microelectronics to the terminals of the thin-film circuit. This novel approach to epidermal electronics allows for the seamless integration of integrated-circuit microchips and other packaged microelectronics that could be used for sensing, signal processing, power regulation, and data transmission. As the materials and fabrication methods for stretchable circuits and epidermal electronics grow more sophisticated, R&D efforts must pursue techniques to integrate these circuits with rigid microelectronics for signal processing, wireless communication, and power regulation.
Robust electrical and mechanical interfacing between soft, thin-film circuits and rigid, packaged electronics is challenging because stress concentrations and the potential for material failure are greatest near regions where there is a mechanical impedance mismatch. Overcoming this challenge is essential for creating soft, sticker-like wireless PSM technologies that maintain digital circuit functionality under bending, stretching, contact pressure, abrasion/rubbing, impact, and other conditions that can arise during TCCC.
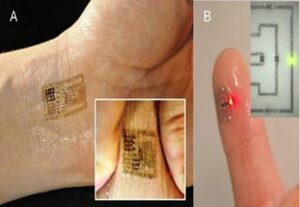
Figure 2. (A) Epidermal electronic circuit for multi-modal physiological sensing [11]. (B) Electronic tattoo with LM-coated conductive ink circuitry [14].
Microelectronics Integration
Materials architectures and manufacturing methods for integrating biosensors thin-film stretchable circuits, and packaged microelectronics stand as an open challenge for the next generation of wearable PSM devices. The complete integration of functional components for wireless biosensors that adhere to the skin (and operate outside of laboratory conditions) has already met with some success. One example is the Bluetooth-enabled PSM system presented in Figure 3A [15]. Here, serpentine copper wiring and an fPCB are used to create a photonic pulse oximetry circuit that conforms to the tip of a finger. The circuit is able to measure the heart rate and blood oxygenation level of a human subject pedaling on a stationary bike.
The circuit is produced using a laser- based rapid prototyping approach in which copper film is patterned with a 355 nm Nd:YAG UV laser micromachining (UVLM) system, and then transferred to a medical-grade adhesive using a selective bonding technique. The circuit is then sealed with a z-axis tape that is conductive through its thickness and allows for via connections between the circuit terminals and surface-mounted electronics. The latter includes a pulseox chip and fPCB with components for battery power and Bluetooth. More recently, a z-axis conductor has been incorporated into a soft and wearable pulseox circuit in which the serpentine copper traces were replaced with gallium-based LM wiring that was also patterned using UVLM [15].

Figure 3. (A) Skin-mounted wireless circuit for pulse oximetry [16]. (B) Flexible glove biosensor with a portable potentiostat mounted to the back of the hand [17].
The integration of a wearable sensor and body-mounted electronics is also presented in Mishra et al. [16], which combines flexible and textile-based sensors for vapor-phase detection of organophosphorus agents. In this implementation, the biosensing circuit is connected to a portable potentiostat attached to the back of the hand (Figure 3B). The potentiostat performs electrochemical characterization and wirelessly transmits the readings to a smartphone. Whereas the integrated system reported by Bartlett et al. [17] functions like a data skin or electronic sticker, the device in Mishra et al. [16] is described as a “lab-on-a-glove” that combines wireless electronics and wearable biosensing with electrochemical analysis.
Conclusions
Compared to the ruggedized portable medical devices currently used in TCCC applications, the wireless PSM sticker technologies presented above are in a nascent stage of development. Nevertheless, recent years have witnessed promising advancements in the merging of soft materials engineering with microfabrication and microelectronics. This has enabled the creation of integrated, wireless, and skin-adhesive biosensing PSM technologies. Further progress may require more robust methods for interfacing stretchable circuitry with rigid microelectronic components. Another challenge for next-generation wearables is the provision of electrical power.
This is especially true in prolonged field care scenarios, in which a sensor must remain operational for up to 72 hours. One promising approach is to use alternative energy sources, like the biofuel cell introduced in Garcia et al. [18] to power a sensor for sweat lactate monitoring. Innovations like these represent an exciting step toward next-generation PSM technologies capable of supporting prolonged operation with limited maintenance or user intervention.
As the material architectures for wireless PSM stickers mature, future efforts should seek to improve the algorithms that translate biosensor readings into actionable data. Such algorithms must account for variations in sensor placement on the body, physical activity, and individual physiology. Compared to conventional medical equipment, wireless electronic stickers enabled with continuous real-time monitoring introduce challenges related to perspiration, changes in physical activity and environment, and motion artifacts.
Addressing these challenges will require extensive clinical and field studies in order to source data, perform expert-supervised classification, and create training sets that can be incorporated into learning-based algorithms for diagnostics and decision-making. Integrating PSM technologies into TCCC practice may also require the eventual replacement of civilian wireless standards and data management tools with Department of Defense-approved systems.
References
1. Montgomery, R. R., & Anderson, Y. L. (2016, March). Battlefield medical network: Biosensors in a tactical environment (Master’s thesis). Monterey, CA: Naval Postgraduate School. Retrieved from http://www.dtic.mil/dtic/tr/fulltext/u2/1027506.pdf
2. Looney, D. P., Buller, M. J., Welles, A. P., Leger, J. L., Stevens, M., Gribok, A. V., Hoyt, R. W., & Rumpler, W. V. (2017, May). Accuracy of ECTemp models in predicting core temperature and circadian rhythm indicators from heart rate: 1600 Board #275 June 1 900 AM – 1030 AM. Medicine & Science in Sports & Exercise, 49(5S), 454. doi:10.1249/01.mss.0000518130.78412.af
3. Aviña, G., & Nichols, G. (2018, March 22). Wearables for physiological monitoring and the DoD [Webinar]. Homeland Defense & Security Information Analysis Center. Retrieved from https://www.hdiac.org/webinar/ hdiac-webinar-wearables-for-physiological-monitoring-and-the-dod/
4. Metu, S., Sadler, L., & Winkler, R. (2015). Wearable notification via dissemination service in a pervasive computing environment (Rep. No. ARL-TR-7449). Adelphi, MD: Army Research Laboratory, Computational an Information Sciences Directorate. Retrieved from https://www.arl.army.mil/arlreports/2015/ARL-TR-7449.pdf
5. Hirschberg, D. L., Samuels, A. C., Rosenzweig, C. N., Lux, M. W., Emanuel, P. A., Miklos, A. E., . . . Brooks, J. R. (2016, May). Assessment of wearable technology for integrated decision support (Rep. No. ECBC-TR-1377). Fort Belvoir, VA: Defense Threat Reduction Agency. Retrieved from http://www.dtic.mil/dtic/tr/fulltext/u2/1009537.pdf
6. Friedl, K. E., Buller, M. J., Tharion, W. J., Potter, A. W., Manglapus, G. L., & Hoyt, R. W. (2016, March). Real time physiological status monitoring (RT-PSM): Accomplishments, requirements, and research roadmap (Rep. No. TN16-2). Natick, MA: U.S. Army Research Institute of Environmental Medicine. Retrieved from http://www.dtic.mil/dtic/tr/fulltext/u2/a630142.pdf
7. Vigneshvar, S., Sudhakumari, C. C., Senthilkumaran, B., & Prakash, H. (2016, February 16). Recent advances in biosensor technology for potential applications – An overview. Frontiers in Bioengineering and Biotechnology, 4(11), 1–9. doi:10.3389/fbioe.2016.00011
8. Rose, D. P., Ratterman, M. E., Griffin, D. K., Hou, L., Kelley-Loughnane, N., Naik, R. R., . . . Heikenfeld, J. C. (2015, June). Adhesive RFID sensor patch for monitoring of sweat electrolytes. IEEE Transactions on Biomedical Engineering, 62(6), 1457–1465.doi:10.1109/TBME.2014.2369991
9. Gao, W., Emaminejad, S., Nyein, H. Y. Y., Challa, S., Chen, K., Peck, A., . . . Lien, D. H. (2016, January 28). Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature, 529(7587), 509–514. doi:10.1038/nature16521
10. Imani, S., Bandodkar, A. J., Mohan, A. V., Kumar, R., Yu, S., Wang, J., & Mercier, P.P. (2016, May 23). A wearable chemical–electrophysiological hybrid biosensing system for real-time health and fitness monitoring. Nature Communications, 7(11650), 1–7. doi:10.1038/ncomms11650
11. Kim, D-H., Lu, N., Ma, R., Kim, Y. S., Kim, R. H., Wang, S., . . . Yu, K.J. (2011, August 12). Epidermal electronics. Science, 333(6044),838–843. doi:10.1126/science.1206157
12. Lo, J., Lee, D. J. L., Wong, N., Bui, D., & Paulos, E. (2016, June). Skintillates: Designing and creating epidermal interactions. Proceedings of the 2016 ACM Conference on Designing Interactive Systems, 853–864). doi:10.1145/2901790.2901885
13. Weigel, M., Nittala, A. S., Olwal, A., & Steimle, J. (2017, May). SkinMarks: Enabling interactions on body landmarks using conformal skin electronics. Proceedings of CHI 2017 (SIGCHI Conference on Human Factors in Computing Systems), 3095–3105).doi:10.1145/3025453.3025704
14. Tavakoli, M., Malakooti, M. H., Paisana, H., Ohm, Y., Marques, D. G., Alhais Lopes, P., . . . Majidi, C. (2018, July). EGaIn-assisted room-temperature sintering of silver nanoparticles for stretchable, inkjet-printed, thin-film electronics. Advanced Materials, 30(29), 1801852. doi:10.1002/adma.201801852
15. Lu, T., Markvicka, E. J., Jin, Y., & Majidi, C. (2017, June). Soft-matter printed circuit board with UV laser micropatterning. ACS Applied Materials & Interfaces, 9(26),22055–22062. doi:10.1021/acsami.7b05522
16. Mishra, R. K., Hubble, L. J., Martín, A., Kumar, R., Barfidokht, A., Kim, J., . . . Wang, J. (2017, March). Wearable flexible and stretchable glove biosensor for on-site detection of organophosphorus chemical threats. ACS Sensors, 2(4), 553–561. doi:10.1021/acssensors.7b00051
17. Bartlett, M. D., Markvicka, E. J., & Majidi, C. (2016, September). Rapid fabrication of soft, multilayered electronics for wearable biomonitoring. Advanced Functional Materials, 26(46), 8496–8504. doi:10.1002/ adfm.201602733
18. Garcia, S. O., Ulyanova, Y. V., Figueroa-Teran, R., Bhatt, K. H., Singhal, S. and Atanassov, P. (2016). Wearable sensor system powered by a biofuel cell for detection of lactate levels in sweat. ECS Journal of Solid State Science and Technology 5(8), M3075–M3081. doi:10.1149/2.0131608jss


