Background
Homeland defense and security depends on peak performance of field personnel and early detection of exposure to chemical/biological threats. Field-deployable molecular monitoring both on body or in body is finally becoming a believable near-term prospect.
Introduction
Humans continue to be mission-critical components, thus limiting mission efficacy in performance and health. This human-dependence highlights the need to monitor, augment, and intervene in human performance. The importance of human monitoring is not new. Six decades ago, despite their reluctance, the astronauts in the U.S. Apollo missions were monitored with a battery of electrodes to collect electrocardiograms (heartbeat waveforms), a heated thermistor to detect breathing, and a rectal temperature probe (Figure 1).
![Figure 1. Wearable Biosensor Kit Used in the Apollo Space Missions to Track Astronaut Vitals (Source: National Air and Space Museum [1]).](https://hdiac.dtic.mil/wp-content/uploads/2024/11/Heikenfeld-figure-1.jpg)
Figure 1. Wearable Biosensor Kit Used in the Apollo Space Missions to Track Astronaut Vitals (Source: National Air and Space Museum [1]).
After these early breakthrough successes, the advancement of wearable monitoring has been slower and somewhat evolutionary. Conveniently, more wearable health monitoring can be obtained in just a smartwatch; however, the problem persists that these sensors lack specificity [2]. Less specific means exactly how it sounds—the measure is weakly specific to any cause or condition. For example, how many different causes are there for changes in heart rate? If greater specificity for a condition is desired, then molecules inside the body will need to be measured. Most people experience this molecular measurement each year as they are tested for a plethora of molecule concentrations via blood draws at their annual physical exams. U.S. defense experts recognized 20 years ago the importance of high-frequency or continuous molecular monitoring [3]:
Monitoring is necessary to ensure that operational personnel are as physically fit as possible because success on the battlefield is to a great extent dependent on the ability of combat service members to carry and operate weapons, to overcome physical obstacles, to traverse distances in harsh environments, and to endure a host of physical stresses and strains that could easily overwhelm unfit individuals.
However, to maximize both specificity and actionability during a mission, measurement should occur in real time in a simple wearable format. Real-time measurement is now possible with the revolutionary success of continuous glucose monitors for diabetes management (Figures 2c and 3). While implanted biochemical monitors are starting to emerge, for most foreseeable future applications, wearable biochemical monitors will have the greatest utility for most users.
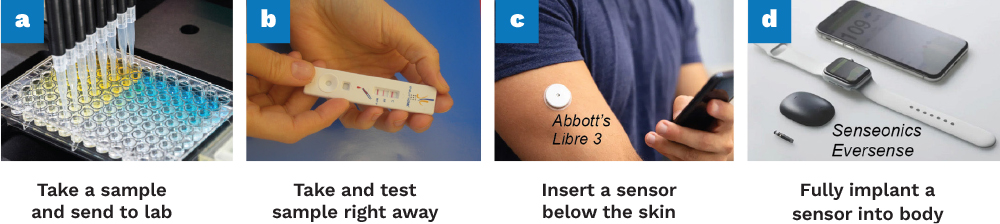
Figure 2. Evolution of Diagnostics Throughout the Decades (Source: J. Heikenfeld).
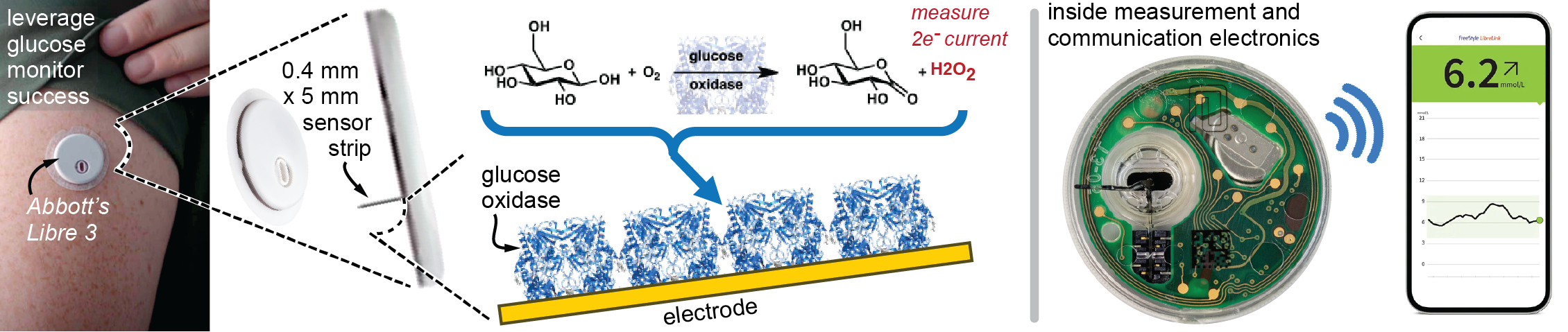
Figure 3. Example Success in Molecular Monitoring With a Specific Example of Abbott’s Libre 3 Glucose Monitoring System (Source: J. Heikenfeld).
However, challenges remain in moving beyond just glucose. This article will address these challenges and cover recent advances showing that after decades of pursuit by the U.S. Department of Defense, wearable molecular monitoring is arguably right around the corner. Once available, some examples of potential outcomes that could raise homeland defense to an entirely new tier of performance include the following [3, 4]:
- Monitor individual health and performance status to optimize individual self-regulation and workload distribution across the team.
- Accurately identify exposure to chemical-biological agents before the symptoms of potential exposure are even suspected and administer medical countermeasures before permanent harm occurs.
- Optimize individual health and performance readiness outside the performance of duty, including enabling faster recovery from strenuous performances.
- Warn the individual and team when performance failure is imminent from physiological, psychological, or environmental overload.
- In training, quickly identify and characterize the individual needs in high-stress and strain situations to prevent washback (loss of trainees).
- Monitor the effects of long-term health risks and associated exposures to stress, strain, and environment.
Don’t Recreate the Wheel
Although the largest challenge for biochemical monitoring is moving beyond the success of glucose monitoring, the wheel does not need to be created. For example, when applying a modern glucose monitor, a simple one-step, spring-loaded, and pain-free insertion method implants a glucose sensor into the skin on a tiny ~5-mm-deep and ~0.4-mm-wide plastic strip. The device automatically activates, allowing diabetes patients to accurately track their glucose levels via Bluetooth communication to their smartphone for up to two weeks of wear time. This has been a resounding success.
Instead of recreating the wheel, advances in glucose monitors by companies such as Abbott, Dexcom, Medtronic, and others should be leveraged. While several emerging alternative monitoring approaches are in development like sweat or other fully nonskin-invasive techniques, these alternatives are unproven and, at best, will be inferior in specificity and accuracy to today’s glucose monitoring approach [5]. Modern-day glucose monitors required decades of work by multiple armies of scientists and engineers, but their development also benefited from a molecule inside the human body called glucose oxidase.
Glucose oxidase only reacts to glucose and allows glucose monitors to find the needle (glucose) in the haystack (a plethora of all the other molecules in blood). To better explain this concept, a good analogy is a “lock” and “key.” Glucose oxidase (the lock) is a unique fit for glucose (the key), which together “open” a chemical reaction that produces peroxide. Peroxide is then electrochemically measured at the electrode surface as a current (Figure 3). Simply put, more glucose ⟶ more peroxide ⟶ more electrical current. However, this lock and key by design only works for glucose—so how are other molecules measured?
Finding Other Needles in the Haystack
Measurement specificity for molecules is, at first glance, a very difficult challenge to overcome. For example, how can a sensor be developed that can distinguish cholesterol (a waxy sterol) from estrogen (female sex hormone) from testosterone (male sex hormone) from cortisol (stress hormone) given that they are all structurally very similar (Figure 4)? This is where >99% of the biosensors demonstrated in a beaker in academic journals will fail in the real-world use because their sensor surface when touching biofluid in the body interacts with far more than just what they are trying to measure. They suffer from being nonspecific. How can specificity issues be circumvented to move forward?
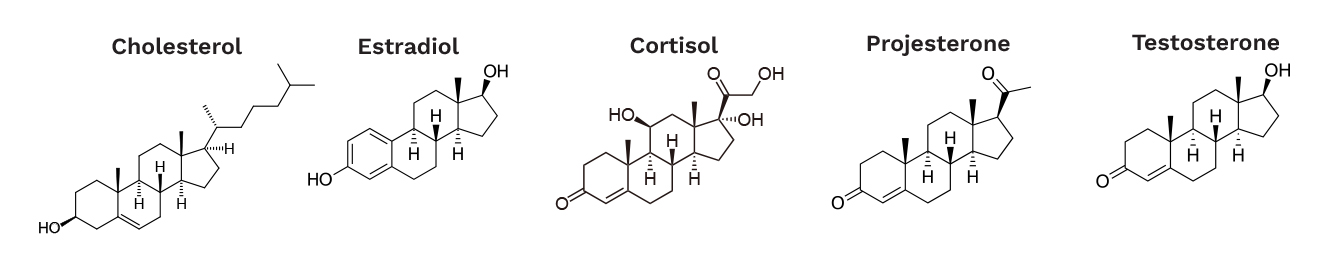
Figure 4. Steroid Hormones Circulating in the Body (Source: J. Heikenfeld).
Enzymes are one way to solve this problem. Glucose oxidase used in the monitor shown in Figure 2 is made up of 604 amino acids. There are 20 different amino acids used to build proteins and enzymes. Having n=tens to hundreds of amino acid combinations with 20 different types of amino acids adds up to 20n options, which seems like an infinite number of locks (the sensor) to make to fit a key (the molecule). While the number of potential amino acid combinations is seemingly limitless, creating a molecule that causes a specific enzymatic reaction is quite difficult and cannot be easily engineered in a lab. Furthermore, the enzymatic approach is limited to high-concentration molecules such as glucose, lactate, ethanol, and ketones, which are around millimolar concentrations (thousandths of a mole in a liter of solution).
In contrast, most of the high-importance molecules to measure are hormones and proteins, which are at nanomolar to picomolar concentrations—this is a million to a billion times lower than glucose [6]. The only way to resolve this problem is to mimic the body’s own molecular signaling mechanisms by using tunable molecular-affinity based interactions as the lock and key.
A simple example of affinity binding is cortisol, which is a hydrophobic (water disliking) steroid hormone (Figure 4) that binds to cortisol receptors in the body. These cortisol receptors contain, at least in part, hydrophobic amino acids enabling favorable hydrophobic interactions with cortisol (disliking being in water). As cortisol concentrations increase in the body, there is more cortisol in the blood (in water), thus increasing the chance for it to interact with its receptor. Therefore, when a concentration goes above the nanomolar range, cortisol begins to bind to its receptor. More cortisol, more receptor binding—less cortisol, less receptor binding, which typically occurs over a measurable range of ~100×. The body itself achieves incredible specificity for cortisol vs. similar molecules (Figure 4) by using different sequences of amino acids. Leveraging different sequences of amino acids for affinity binding is how antibodies specifically find their target in the body. Mother nature had over a billion years to slowly develop these perfect lock amino acid sequences for specific keys—time that cannot be afforded to develop biochemical monitoring for homeland defense applications. How can selecting the right lock for the key (molecule) be expedited?
Fortunately, there is a way to quickly engineer these lock-and-key interactions for most types of interest analytes. The trick is to use short, single-stranded DNA called aptamers. The good thing about aptamers is that anyone can now purchase a large library of aptamer molecules with random sequences of nucleotides (A, T, C, or G, or manmade options) instead of different amino acids like with proteins and enzymes. For example, once a standard library of 1015 different aptamer sequences is obtained, a process called SELEX can be used to wash these aptamers (different types of locks) over the target molecule (the key). This process finds the possible lock-and-key fits, uses DNA amplification to amplify the best fits, and then repeats for several rounds until a few aptamer sequences are obtained that specifically match the target molecule. Does this really work in practice? Absolutely, considering that commercially SomaLogic now sells an assay based on this technology that can quantify >11,000 human proteins from a single, tiny, 55-µL blood sample (i.e., 1/50th of a teaspoon of blood to simultaneously measure 1/3 of the human proteome).
SomaLogic’s lock and key for the important inflammation monitoring protein, IL-6, is shown in Figure 5. The purple parts of the aptamer are manmade nucleotides with greater hydrophobicity. These more hydrophobic, manmade nucleotides on the aptamer dislike water and are attracted to the hydrophobic amino acid portions of the IL-6 protein. Other companies like Aptamer Group, Aptagen, and Aptus Biosciences are realizing the power of these manmade nucleotides and offering them commercially.
![Figure 5. An Exquisite Lock-and-Key Fit of an Aptamer to an Inflammatory Protein Like IL-6 (Source: SomaLogic 2023 [7]).](https://hdiac.dtic.mil/wp-content/uploads/2024/11/Heikenfeld-figure-5.png)
Figure 5. An Exquisite Lock-and-Key Fit of an Aptamer to an Inflammatory Protein Like IL-6 (Source: SomaLogic 2023 [7]).
Pulling It All Together
How can a single measurement assay technology (Figure 2a) like SomaScan be converted into a continuous measurement sensor (Figure 2c)? In 2005, Kevin Plaxco and colleagues at the University of California Santa Barbara found that by attaching a redox active molecule like methylene blue to one end of the aptamer and using the opposite end to attach the aptamer to a gold electrode, these powerful lock-and-key mechanisms can be measured electrically [8]. As shown in Figure 6, as the molecule of interest binds to the aptamer, the aptamer changes shape, which moves the methylene blue closer to or farther from the gold electrode. As the methylene blue molecule moves closer to the electrode, greater redox electron exchange occurs with the electrode; therefore, greater current is measured. These current changes can then be easily correlated with measured molecule concentration. Because lock-and-key binding between the aptamer and measured molecule is reversible and requires no other reagents (like assays require), this allows continuous monitoring in the body.
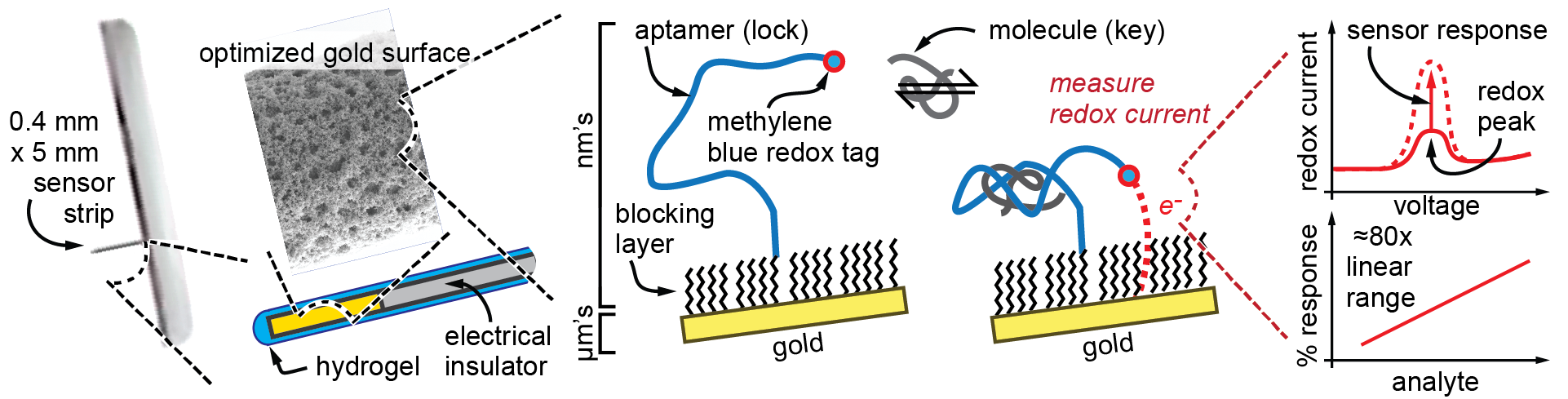
Figure 6. Adapting Aptamer Sensors Onto Continuous Glucose Monitoring Device Formats (Source: J. Heikenfeld).
If aptamer sensor technology were available in 2005, why is significant commercial progress just now being seen? The single biggest challenge that hindered aptamer sensor development was its fragility, which only permitted short-term tests using surgically implanted sensors in animals [9].
Results and Discussion
Figure 7 shows some of the recent breakthroughs that will make performance and health monitoring a reality. In the past four years, through corporate, U.S. Air Force and Space Force (Patrick Bradshaw and Steve Kim), Office of Naval Research, National Science Foundation, and National Institutes of Health support, the coauthors and their affiliated organizations have demonstrated the following:
- Aptamer sensor stability for multiple months in whole serum (Figure 7a) exceeding even the two-week commercial benchmark established for glucose sensors. This longevity is an impressive achievement for aptamer sensors measured in a biofluid like that found in the body.
- Batch fabrication of these sensors with uniform performance across the entire batch, enabling factory calibration (i.e., make hundreds of sensors, calibrate a small subset at the factory, and use that calibration data with software to provide accurate operation for the uncalibrated sensors in real-world use).
- Most importantly, the sensors are fabricated on tiny hair-sized plastic electrode strips that match the dimensions and materials used in commercial glucose monitors. Therefore, the sensors can leverage the proven device architectures for glucose monitors (Figures 2c and 3), including single-step and pain-free, nonsurgical sensor insertion into the skin.
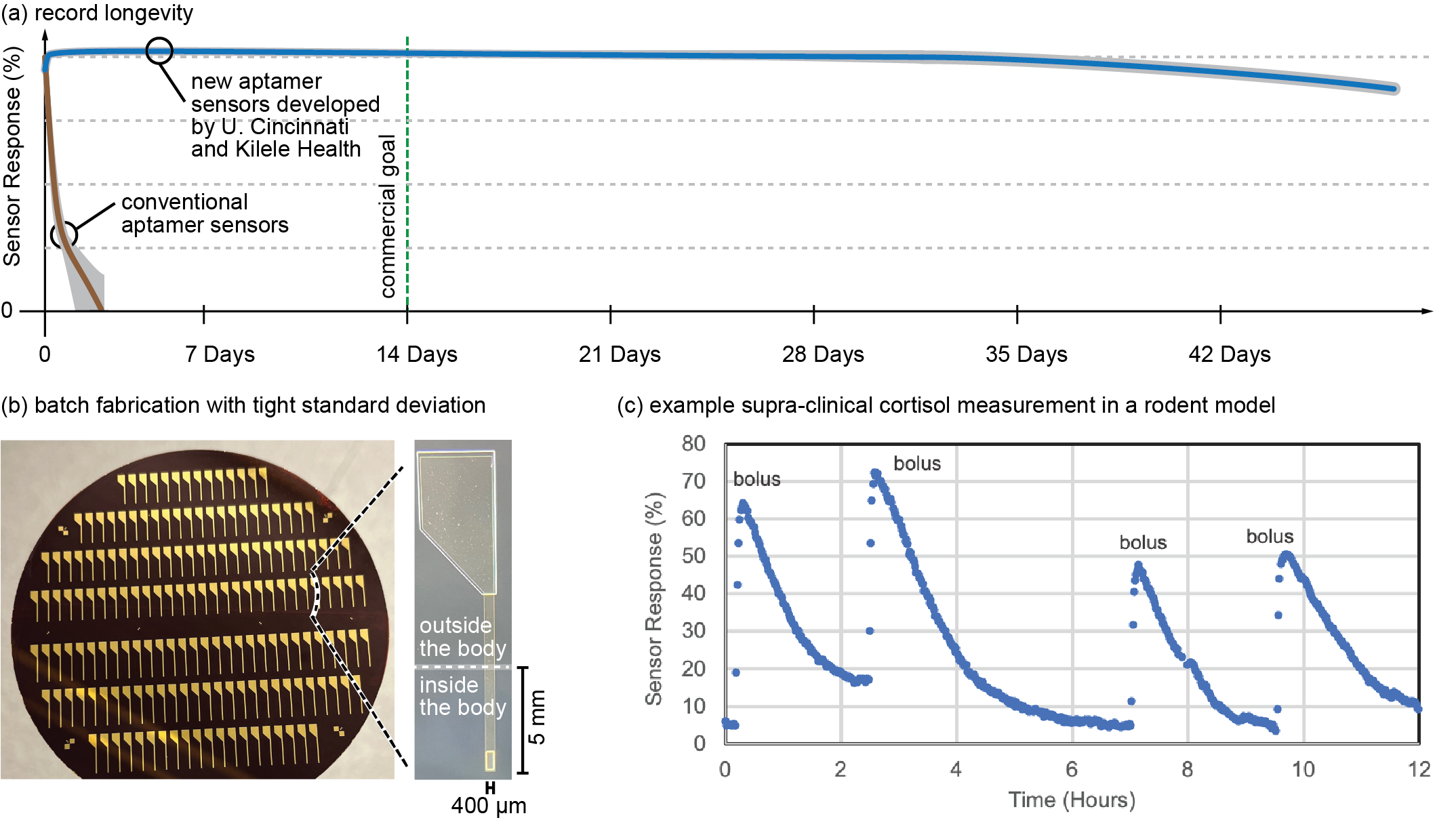
Figure 7. Reasons to Believe that Aptamer Sensors Are Ready to Enable Molecular Monitoring Beyond Glucose (Source: J. Heikenfeld).
Two equally important areas of work remain before commercial deployment of these improved aptamer-based molecular monitors. The first requires commercialization efforts to translate these research and development innovations into a real product. This process involves numerous safety and reliability checks, manufacturing yields, and other issues that will require time and effort. Fortunately, the pioneering progress that glucose monitors have already demonstrated will de-risk many of these translational steps. The University of Cincinnati and Kilele Health Inc. are laser focused on this strategy of adapting glucose monitor technology for use with aptamer sensors, a strategy that surprisingly most others have not adopted [5].
The second area of important work requires figuring out the best molecules to target. This is where the U.S. Department of Defense (DoD) requires more fundamental work to identify what molecules will best address the goals of the example applications listed and discussed in other reviews on human performance by Lee et al. [6]. Team members from the University of Cincinnati and Kilele Health Inc. are currently partnered with organizations like the Institute for Human and Machine Cognition to identify those next-level biomarkers [10]. Some long-discussed molecules such as cortisol (Figure 7c) and inflammation molecules may be early targets and will be critical to monitoring mental and physical stress status and resiliency.
It has been a long road for the DoD in pursuing wearable molecular monitoring beyond glucose. Now is the time to seize the opportunity, as aptamer-based molecular monitors provide an alternative to glucose by showing robust performance for multiple weeks with a commercially proven glucose monitor format. If all goes according to publicized commercialization plans, early applications of such sensing technology may begin to appear in medical applications in 2027, with homeland defense applications following shortly thereafter.
References
- National Air and Space Museum. “Inventing Apollo Spaceflight Biomedical Sensors.” https://airandspace.si.edu/stories/editorial/inventing-apollo-spaceflight-biomedical-sensors, 2016.
- Heikenfeld, J., A. Jajack, J. Rogers, P. Gutruf, L. Tian, T. Pan, et al. “Wearable Sensors: Modalities, Challenges, and Prospects.” Lab Chip, vol. 18, no. 2, 2018.
- Institute of Medicine (U.S.) Committee on Metabolic Monitoring for Military Field Applications (IMCMMMFA). “Monitoring Metabolic Status: Predicting Decrements in Physiological and Cognitive Performance.” Washington, D.C.: National Academies Press; Rationale for Military Interest and Current Capabilities in Monitoring Metabolism, https://www.ncbi.nlm.nih.gov/books/NBK215709/, 2004.
- Sirikupt, C., G. Wauters, and T. Kaloudiotis. “The Future of Biosensors in European Defence Web.” Finabel, https://finabel.org/wp-content/uploads/2020/03/FFT_The_Future_of_Biosensors_in_European_Defence_Web.pdf, 2020.
- Heikenfeld, J. “Please Learn from My Mistakes: The Acute Need for an Entrepreneurial Mindset in Academic Biosensor Research.” Front Sensors, vol. 5, https://www.frontiersin.org/journals/sensors/articles/10.3389/fsens.2024.1408158, 2024.
- Lee, E. C., M. S. Fragala, S. A. Kavouras, R. M. Queen, J. L. Pryor, and D. J. Casa. “Biomarkers in Sports and Exercise: Tracking Health, Performance, and Recovery in Athletes.” J. Strength Cond. Res., vol. 31, no. 10, pp. 2920–2937, October 2017.
- SomaLogic. “SomaScan® Assay v4.1.“ https://somalogic.com/wp-content/uploads/2023/03/SomaScan-Assay-v4.1-Technical-Note.pdf, 2023.
- Xiao, Y., A. A. Lubin, A. J. Heeger, and K. W. Plaxco. “Label-Free Electronic Detection of Thrombin in Blood Serum by Using an Aptamer-Based Sensor.” Angew. Chem. Int. Ed. Engl., vol. 44, no. 34, pp. 5456–5459, 26 August 2005.
- Arroyo-Currás, N., P. Dauphin-Ducharme, K. Scida, and J. L. Chávez. “From the Beaker to the Body: Translational Challenges for Electrochemical, Aptamer-Based Sensors.” Analytical Methods, vol. 12, no. 10, pp. 1288–1310, http://dx.doi.org/10.1039/D0AY00026D2020, 2020.
- Institute for Human and Machine Cognition (IHMC) – USAF Prime Award FA9550-23-1-0661. “Multidimensional Modeling of Stress Resilience for Robust Space Force Guardian Performance.” PI Marcas Bamman, 2023.
Biographies
Jason Heikenfeld is a professor at the University of Cincinnati and chief technology officer at Kilele Health Inc. At the University, he directs the Novel Devices Laboratory, an internationally leading research lab in biosensing technology. Dr. Heikenfeld holds a bachelor’s degree in electrical engineering and a Ph.D. in electrical engineering from the University of Cincinnati.
Aleksandar Karajic is the principal scientist at Kilele Health Inc., where he develops wearable electrochemical aptamer sensors to continuously monitor various bioanalytes. His work has focused on wearable electrochemical sensors, electrocatalysis, biofuel cells, and material science. Dr. Karajic holds an M.S. in chemistry and an MSc in analytical and electroanalytical chemistry from the University of Belgrade and a Ph.D. in physical chemistry (electrochemistry) from the University of Bordeaux.
Zach Watkins is a research affiliate at the University of Cincinnati in the Novel Devices Laboratory, where he has focused on integrating electrochemical aptamer sensors into point-of-care and continuous wearable biosensing platforms, with an emphasis on extending sensor operational lifetimes for multiweek use. Mr. Watkins holds a bachelor’s degree in biomedical engineering from North Carolina State University and a Ph.D. that focused on integrating electrochemical aptamer sensors into point-of-care and continuous wearable biosensing platforms from the University of Cincinnati.
Thomas Young is a biomedical engineering Ph.D. student at the University of Cincinnati in the Novel Devices Laboratory. His dissertation research focuses on the feasibility of continuous protein monitoring using electrochemical aptamer sensors.


