Military personnel deployed around the world require high-quality drinking water, and specific procedures are in place to ensure that military field water supplies are properly treated and monitored [1]. Chemical contaminants pose potential threats to field drinking water [2], and although current water treatment technology is highly efficient, removal of contaminants may be inadequate if chemicals are present in source waters at high concentrations or if the chemicals are introduced after processing. In the Army, preventive medicine (PM) personnel periodically
test water supplies using the Water Quality Analysis Set-Preventive Medicine (WQAS-PM) and associated equipment, including field tests for a limited number of specific chemicals of concern. While water supplies can be evaluated more thoroughly at off-site laboratories, these evaluations are done infrequently and are more expensive. Additionally, analytical test results are usually not available for days or weeks.
There is a need, therefore, for field test methods that provide rapid assessment for a broad range of chemical contaminants, including agricultural and industrial chemicals, both organic and inorganic. Analytical chemistry instrumentation for this purpose (e.g., gas and liquid chromatography and mass spectrometry) tends to be complex and expensive, and has limited potential for field use. An alternative approach is to measure the response of a biological indicator to the presence of toxic chemicals in a water sample. Although there are analyte-specific biosensors (e.g., those that use antibodies, enzymes, and nucleic acids for detection of particular chemicals in water [3-6]), many biosensors would be required to detect a broad range of potential contaminants [7]. The goal is to develop a toxicity sensor with biological components that can rapidly respond to a broad spectrum of toxic compounds at concentrations relevant to warfighter health.
The Environmental Sentinel Biomonitor System
At the U.S. Army Center for Environmental Health Research, we developed a toxicity detection device, the Environmental Sentinel Biomonitor system (ESB), based on Army requirements defined in 2013 in a Capability Development Document (CDD) [8]. The Army needs a device that can detect a broad spectrum of toxic industrial chemicals, has field-portability, and utilizes biological components that have a shelf-life of at least nine months (refrigeration, but not freezing, of perishable components is permitted). Finally, the required system must perform satisfactorily
in an independent laboratory evaluation using procedures developed for the U.S. Environmental Protection Agency’s Technology Testing and Evaluation Program (TTEP) [9]. Here, we demonstrate that the ESB meets these requirements, and report for the first time the positive results of an in-theater field evaluation of the ESB system in Iraq and Kuwait during the spring of 2016.
Because of the large number of available toxicity sensor devices and the constraints imposed by Army field use requirements, we used a formal decision analysis approach for toxicity sensor down selection [10]. After considering 38 toxicity sensor technologies, the best performing sensors were found to be a cell-based electric cell-substrate impedance sensing (ECIS) test (Nanohmics, Inc.) and an enzyme inhibition test (ACE™ Rapid Test for Acetylcholinesterase Inhibitors, ANP Technologies) [10]. Together, these toxicity sensors respond to the broadest range of chemicals while minimizing the technical complexity, size, weight, and cost of the system.
The ECIS test monitors alterations in the electrical impedance of a monolayer of rainbow trout gill epithelial cells (RTgill-W1) [11], grown on fluidic biochips to indicate chemical-induced toxicity. Cells are seeded in two separate fluidic channels on the biochip containing electrodes; during testing, one channel receives the control sample and the other receives the test sample. When a toxic chemical compromises the integrity of the cell monolayer, the ECIS sensor records a change in electrical impedance. Water sample toxicity is indicated when the impedance in the control channel differs significantly from the test channel during a one-hour exposure. ECIS biochips can be maintained for at least nine months at 6 degrees Celsius with no media replacement.
The ECIS test is used together with theACE™ test to evaluate a water sample. The ACE™ test is an enzymatic assay designed to detect neurotoxicants—specifically, cholinesterase-inhibiting compounds such as organophosphate and carbamate pesticides. It monitors the activity of the enzymes carboxyl esterase (CE) and acetylcholinesterase (AChE) using a reporting chemical that fluoresces under ultraviolet (UV) light [12].
During testing, control and test water samples are added to separate vials containing either AChE or CE for 30 minutes. Then, the sample-enzyme mix is added to a test ticket well containing the reporting chemical that fluoresces after being cleaved if the active enzyme is present. The ticket is read after 15 minutes; interference with enzyme function will result in detectable color differences. Consumables are stable for at least nine months at room temperature. Equipment for conducting both ECIS and ACE™ tests is stored in the ruggedized ESB carrying case (see Figure 1). The ECIS fluidic biochips, which require temperature control for long-term viability, can be transported in small insulated containers.
ESB Testing and Evaluation
To ensure that the ESB meets Army requirements as defined in the CDD, and is useful to Army PM personnel, we conducted three types of testing: toxicity testing to determine ESB responses to challenge chemicals, environmental testing, and field evaluations of ESB performance.
Toxicity Testing
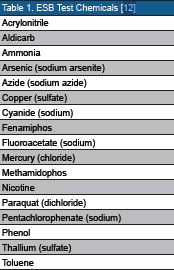
To meet Army requirements, the ESB needs to detect at least 50 percent of a diverse set of 18 chemicals within two hours (see Table 1). For each chemical, the ESB needs to respond to water sample concentrations that exceed the seven- to 14-day military exposure guideline (MEG) levels, assuming an individual water consumption rate of 15 L per day, which is typical of arid environments [13]. The upper limit for a useful toxicity sensor response is defined as the estimated human lethal concentration (HLC) [14]; a level estimated to be lethal to a 70 kg person consuming 15 L of water in a day. Testing results showed that the combined ACE and ECIS sensor responses exceed CDD requirements for toxicant response to the 18 chemicals in the test set (i.e., they were able to detect more than 50 percent of the chemicals within one hour at levels that were relevant to human health) [12, 15]. It is important to note that while these test chemicals include diverse substances with varying modes of toxic action, they are not the sole focus for ESB detection; rather they are a subsample of a much larger set of potential threat chemicals to which the ESB can respond.
In addition to the toxicants, potential interfering materials were evaluated. For Army field water, the primary concern is contamination by chemicals used to disinfect drinking water— namely, chlorine and chloramine. For chloramine, the ECIS responds at 10 mg/L, with no response at 5 mg/L [15]. However, in practice, water samples for ESB testing can be dechlorinated prior to ESB testing using a reducing agent, so drinking water disinfection chemicals should not be an issue.
Environmental Testing
Army medical equipment, such as the ESB system, is required to pass field-relevant environmental testing administered by the U.S Army Medical Research and Materiel Command Test Branch, following Military Standard (MIL-STD) 810G [16]; these tests were conducted on both the ACE and ECIS instruments [17]. “Operational” refers to active testing with the ACE and ECIS under the specified test conditions. “Non-operational” refers to storage under the specified test conditions, with testing done at room temperature.
- Low temperature, operational (15° C) and non-operational (0° C)
- High temperature, operational (45° C) and non-operational (71° C)
- Altitude (4,572 meters equivalent), operational and non-operational
- Settling dust and sand (settling for one hour on exposed equipment), non-operational
- Ground vibration (1.90 root mean square acceleration (Grms) for one hour), non-operational
- Transit drop (ESB packaged in its case was dropped from a height of four feetonto a steel surface)
- Loose cargo transportation, non-operational (ESB packaged in its case was vibrated at 300 rpm, 2.5 cm amplitude for one hour)

Figure 1. ECIS (instrument on the left) and
ACE™ sensors (right) with reusable supplies
packaged in the ESB carrying case.
Both the ECIS and ACE tests passed environmental testing [17]. One notable point was the low temperature operational ACE test conducted, where the negative control tickets failed to show viable enzyme activity after 15 minutes. This was likely due to reduced enzyme activity at the lower test temperatures. Test readings were successful when the incubation time was extended to 25 minutes, meeting the standard of a successful test.
Field Testing
The ESB system was evaluated using a customer assessment organized by U.S. Army Medical Department Board, as well as in field assessments by PM personnel. The customer assessment determined if the ESB system would support the PM mission and if it was usable in an operational environment [18]. Following a four-hour familiarization period with the equipment, six teams of Army PM specialists and an environmental science and engineering officer used the ESB system in an operational environment to process blind water samples that were either negative or positive controls.
Operation was tested using commercial power (120 volts AC [VAC]), generator power (220 VAC), and internal battery power. U.S. Army Medical Department Board staff then evaluated ESB system performance against operationally-relevant criteria.
Overall, the results were supportive of ESB field use [18].The functionality of the ESB system supports the PM mission. Test completion time met CDD requirements, and 11 of 13 assessment participants indicated that ESB functionality was mission supportive.
- The ESB system was usable in an operational environment. System weight met CDD requirements, and the ESB functioned under 120 VAC, 220 VAC, and internal battery power as required.
All 13 assessment participants thought the ESB was usable for water sample analysis in an operational environment. - The ESB does not pose any actual or potential safety hazard to personnel, equipment, or facilities in an operational environment. The pliers used to open the ACE reagent vials pose a minor cut
hazard. - The ESB system was usable after transport. The ESB was used successfully to conduct water sample analyses after a transportation test (see Figure 2).
One identified problem involved several instances of false positive and negative ECIS readings. The false negatives occurred with the ammonia positive control, most likely because the ammonia concentration used was too low. Subsequently, a new positive control test chemical was used.
The false positives were thought to be due to the sensitivity setting used for the system statistical software. As a result, the statistical models were adjusted to incorporate the additional variability noted under field conditions and validated to ensure that no substantial loss in toxicant sensitivity occurred due to the sensitivity adjustments. As a check, PM personnel at the 1st Area Medical Laboratory, Aberdeen Proving Ground, conducted additional ECIS testing. When the ECIS results were analyzed using the refined statistical algorithm, there were no false positive or negative results.
The ESB system (both ECIS and ACE) was also evaluated by the 227th Medical Detachment (Preventive Medicine) in a deployed environment at Taji, Iraq and Camp Arifjan, Kuwait, over the spring of 2016. Two ESB units, along with consumables, were shipped from the U.S. to Kuwait. The theater biochemist coordinated evaluation by 18 participants (PM personnel), who completed a total of 20 water tests (eight ECIS and ACE tests at Camp Taji; 12 ECIS tests at Camp Arifjan). Participants were asked to fill out customer assessment forms evaluating their experiences with the ESB system.
Overall, the in-theater evaluations of the ESB system were highly favorable. There were no issues shipping equipment and reagents into theater (five days from Fort Detrick to Camp Taji, Iraq), and ECIS biochips remained viable when maintained by the theater biochemist for all of the evaluations by PM personnel. ECIS tests were completed in 19 of 20 evaluated with only one test yielding an error; there were no false positives. All 20 evaluators felt that the ESB system was supportive of the PM mission. Theater biochemists on different tours also transported the ESB system with them on missions to Camp Taji and missions to Northern Iraq, where successful water testing was performed.
Conclusion
As the ESB developed into a dual toxicity sensor that is currently in the pipeline for Army field deployment, customer assessment and field testing provided critical feedback that could not be obtained in a laboratory setting. Customer input led to modifications in some of the practical aspects of the ESB, such as manipulating pipets and syringes, accurately measuring water samples, ease of opening vials, and clarification of instructional materials. The feedback also allowed for fine-tuning of statistical algorithms to ensure that false negatives and positives would be a rare occurrence. The ESB is scheduled for fielding in FY19 as part of the WQAS-PM to provide a rapid test for chemical-related toxicity in Army field drinking water supplies. As the ESB evolves, we will continue to seek field testing for any new modifications. Although the ESB was initiated specifically to help Army PM personnel screen potable water for the presence or absence of a wide range of toxic chemicals, the fact that all services have a common mission to provide warfighters with clean drinking water supplies means the ESB has applicability across the DoD.
Further, with the potential for development of novel chemical warfare agents or for the introduction of unknown or unsuspected materials into water supplies, the use of a toxicity- based approach for detection of these materials can provide an important complement to currently-utilized analytical chemistry devices.



Figure 2. Soldiers performing water testing with the ESB device. Soldiers used the ESB during the customer assessment in a field laboratory
setting as well as in the back of a High Mobility Multipurpose Wheeled Vehicle after transport on an off-road course.
Acknowledgements
The authors would like to thank Nanohmics, Inc., and ANP Technologies for their commercial support in developing the ESB system. And a final thanks to all of the Army medical personnel, military, civilian, and contractors who have helped in the development and advancement of the ESB system.
Disclaimer
The views, opinions, and/or findings contained in this article are those of the authors and should not be construed as official Department of the Army position, policy, or decision, unless so designated by other official documentation. Citations of commercial organizations or trade names in this article do not constitute an official Department of the Army endorsement or approval of the products or services of these organizations. This research was supported in part by an appointment to the Research Participation Program at the U.S. Army Center for Environmental
Health Research administered by the Oak Ridge Institute for Science Education through an interagency agreement between the U.S. Department of Energy and USAMRMC.
References
1. Departments of the Army, Navy and Air Force. (2010). Sanitary Control and Surveillance of Field Water Supplies. TB MED 577, NAVMED P-5010-10, AFMAN 48-138_IP. Washington, DC.
2. Salem, H., Whalley, C. E., Wick, C. H., Gargan, T. P., & Burrows, W. D. (2008). Chemical warfare agent threat to drinking water. Chemical warfare agents: Chemistry, pharmacology, toxicology, and therapeutics (second edition) (pp. 21-50), Boca Raton, FL: CRC Press/Taylor & Francis Group, LLC.
3. Eltzov, E., & Marks, R. S. (2010). Wholecell aquatic biosensors. Analytical and bioanalytical chemistry, 400(4), 895-913. doi:10.1007/s00216-010-4084-y
4. Pancrazio, J. J., Whelan, J. P., Borkholder, D. A., Ma, W., & Stenger, D. A. (1999). Development and application of cell-based biosensors. Annals of biomedical engineering, 27(6), 697-711.
doi: 10.1114/1.225
5. States, S., Scheuring, M., Kuchta, J., Newberry, J., & Casson, L. (2003). Utility- based analytical methods to ensure public water supply security. Journal – American water works association, 95(4),103-115. doi:10.1002/j.1551-8833.2003.tb10337.x
6. Kelly, T., Baxter, W., McCauley, M., & E. Koglin. (2008). Testing of screening technologies for detection of toxic industrial chemicals in all hazards receipt facilities. (EPA/600/R-08/034). Washington, DC: U.S. Environmental Protection Agency.
7. Brennan, L. M., Widder, M. W., Lee, L. E., & van der Schalie, W. H. (2012). Long term storage and impedance-based water toxicity testing capabilities of fluidic biochips seeded with RTgill-W1 cells. Toxicology in vitro, 26(5), 736-745. doi:10.1016/j.tiv.2012.03.010
8. U.S. Army. (2013). Capability Development Document for Environmental Sentinel Biomonitor Increment: 1. Catalog of Approved Requirement Documents (CARDS #14099). (Unpublished manuscript, available from the Office of the U.S. Army Deputy Chief of Staff, G-8, 700 Army Pentagon, Room 3E406, Washington, DC 20310-0700)
9. U.S. Environmental Protection Agency. (2008). Quality Management Plan National Homeland Security Research Center Technology Testing and Evaluation Program Version 3. Columbus, OH. Retrieved from https://nepis.epa.gov/ Exe/ZyPURL.cgi?Dockey=P10062P1.txt .
10. Army Edgewood Chemical Biological Center, Aberdeen Proving Ground, MD. (2018). Environment Sentinel Biomonitor Technology Assessment. Retrieved from http://www.dtic.mil/dtic/tr/fulltext/u2/a586335.pdf
11. Lee, L. E. J., Dayeh, V. R., Schirmer, K., & Bols, N. C. (2009). Applications and potential uses of fish gill cell lines: examples with RTgill-W1. In Vitro Cellular & Developmental Biology-Animal, 45(3-4), 127-134. doi: 10.1007/s11626-008-9173-2
12. U.S. Army Center for Environmental Health Research, Fort Detrick, MD. (2014). An Evaluation of the NIDS® ACE™ Test. Retrieved from http://www. dtic.mil/dtic/tr/fulltext/u2/a603987.pdf
13. U.S. Army Public Health Command. (2013). Environmental health risk assessment and chemical exposure guidelines for deployed military personnel. Aberdeen Proving Ground, MD. Retrieved from https://phc.amedd. army.mil/PHC%20Resource%20Library/TG230-DeploymentEHRA-and-MEGs-2013-Revision.pdf
14. U.S. Army Center for Environmental Health Research, Fort Detrick, MD. (2006). Derivation of Human Lethal Doses. Retrieved from http://www.dtic.mil/dtic/tr/fulltext/u2/a494706.pdf
15. Widder, M. W., Brennan, L. M., Hanft, E. A., Schrock, M. E., James, R. R., & Schalie, W. H. (2014). Evaluation and refinement of a field portable drinking water toxicity sensor utilizing electric cell–substrate impedance sensing and a fluidic biochip. Journal of Applied Toxicology, 35(7), 701-708. doi: 10.1002/jat.3017
16. Department of Defense. (2008). Department of Defense test method standard: environmental engineering considerations and laboratory tests. MIL-STD-810G. Retrieved from http://www.wsmr.army.mil/RCCsite/Documents/265-06(Supp)_Radar%20Transponder%20Antenna%20Sys%20Eval%20Handbook/mil-std-810f.pdf
17. U.S. Army Medical Research & Materiel Command Test Branch, Fort Detrick, MD. (2014). Environmental Testing: Environmental Sentinel Biomonitor System. Test File #220.
18. Cabigon, G., & Breckheimer, D. (2014). Customer assessment report for the Environmental Sentinel Biomonitor (USAMEDDBD Project 3-12a). (Unpublished manuscript, available from the U.S. Army Medical Department Center and School, 3630 Stanley Road, Suite 301, JBSA Fort Sam Houston, TX 78234).


